核苷(酸)类似物初治的慢性乙型肝炎患者发生低病毒血症的影响因素及其动态变化分析
DOI: 10.3969/j.issn.1001-5256.2022.12.008
Influencing factors for low-level viremia and their dynamic changes in patients with chronic hepatitis B treated with nucleos(t)ide analogues for the first time
-
摘要:
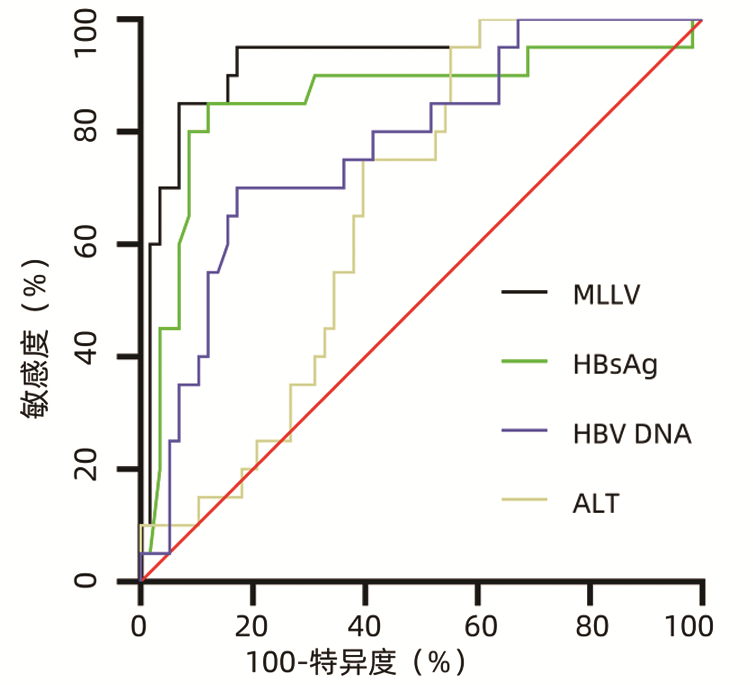
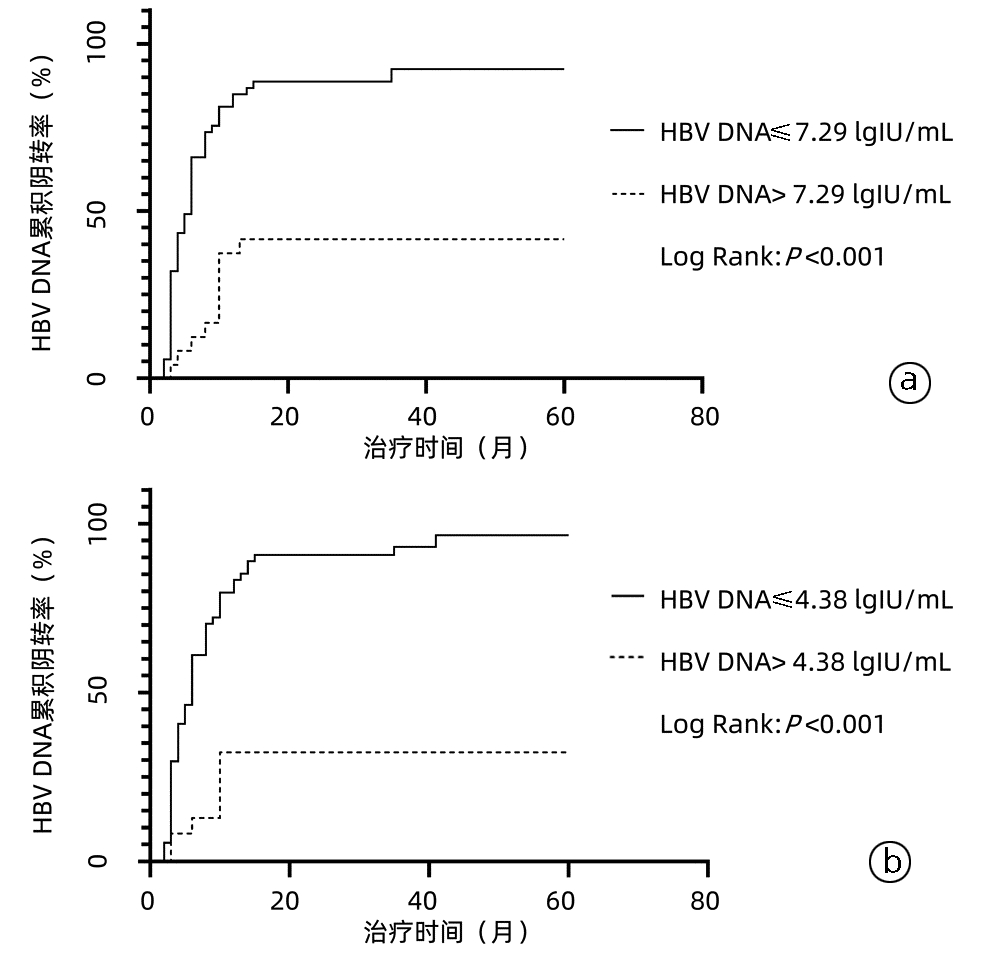
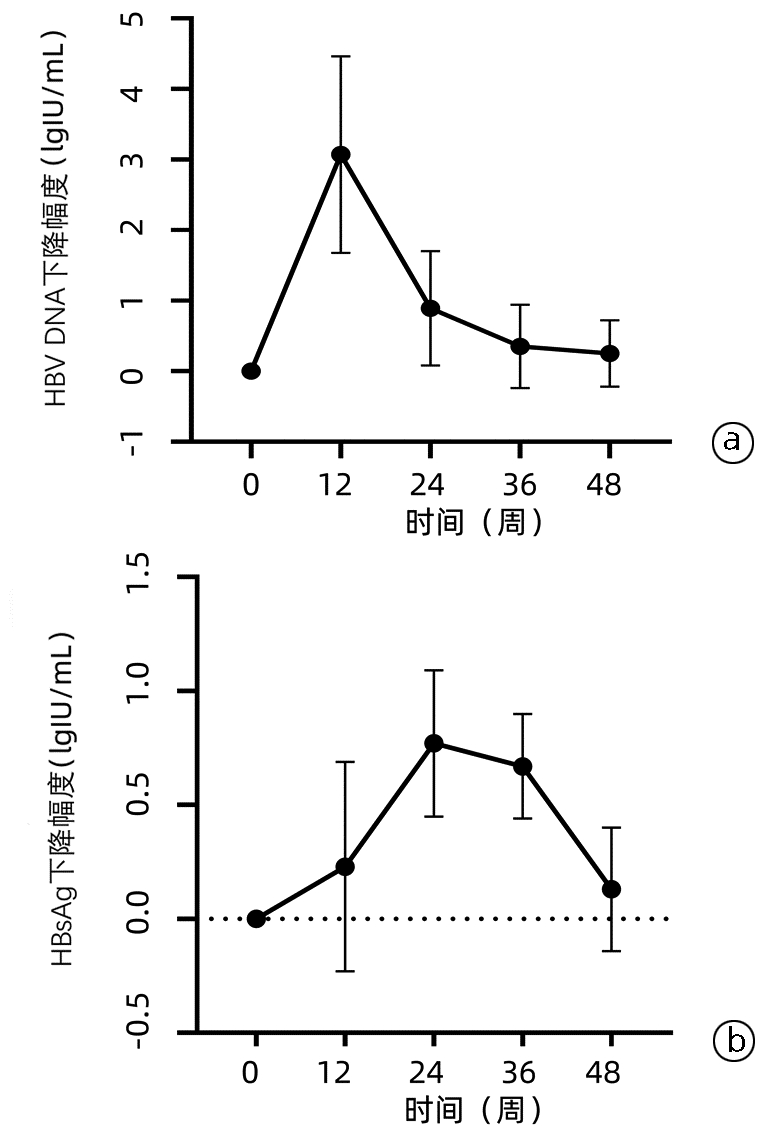
目的 探讨核苷(酸)类似物(NAs)初治的慢性乙型肝炎(CHB)患者发生低病毒血症(LLV)的影响因素,并进一步分析其动态变化。 方法 选取2020年11月—2022年3月于南昌大学第一附属医院感染科门诊就诊且接受NAs抗病毒治疗至少12个月的CHB患者78例,根据治疗期间的HBV DNA水平,将患者分为持续病毒学应答(SVR)组(n=58)和LLV组(n=20)。计量资料两组间比较采用独立样本t检验或Mann -Whitney U检验,计数资料两组间比较采用χ2检验或Fisher精确检验;多因素Logistic回归分析CHB患者发生LLV的独立影响因素,并建立预测模型。采用受试者工作特征曲线(ROC曲线)评价模型的预测价值。使用Kaplan-Meier分析HBV DNA累积阴转率,应用Log-rank检验进行比较。采用重复测量方差分析比较两组间或组内0、12、24、36、48周HBV DNA和HBsAg水平及其变化的差异。 结果 LLV组HBeAg阳性率(90.0% vs 48.3%,χ2=10.701,P=0.001)、HBV DNA log值(7.26±1.46 vs 5.65±1.70,t=-4.178,P<0.001)、HBsAg log值(4.53±0.86 vs 3.44±0.93,t=-4.813,P<0.001)高于SVR组,年龄[29(26~34)岁vs 33(30~43)岁,Z=-2.751,P=0.009]、ALT[67.0(54.0~122.0)U/L vs 111.0(47.0~406.0)U/L,Z=-2.203,P=0.028]、AST[43.5(32.8~62.8)U/L vs 77.5(35.0~213.0)U/L,Z=-2.466,P=0.014]、LSM[7.7(6.3~8.5)kPa vs 8.9(7.2~11.4)kPa,Z=-2.022,P=0.043]低于SVR组。多因素Logistic回归分析显示,基线HBV DNA(OR=2.365,95%CI: 1.220~4.587,P=0.011)、HBsAg(OR=4.229,95%CI: 1.098~16.287,P=0.036)和ALT(OR=0.965,95%CI: 0.937~0.994,P=0.018)是CHB患者发生LLV的独立影响因素;由此建立预测模型Logit(MLLV)=-8.668+1.441×lgHBsAg+0.598×lgHBV DNA-0.016×ALT,其ROC曲线下面积为0.931,高于HBV DNA、HBsAg和ALT(ROC曲线下面积分别为0.774、0.856、0.666),最佳截断值为0.44,敏感度、特异度分别为85.00%、93.10%。基线HBV DNA>7.29 lgIU/mL和HBsAg>4.38 lgIU/mL的CHB患者HBV DNA阴转率明显低于HBV DNA≤7.29 lgIU/mL和HBsAg≤4.38 lgIU/mL的患者(χ2值分别为22.52、26.35,P值均<0.001)。CHB患者的HBV DNA和HBsAg的下降速率分别在第12周和第24周最大,LLV组患者HBV DNA和HBsAg水平在0、12、24、36、48周均高于SVR组(HBV DNA:t值分别为-4.084、-4.526、-5.688、-7.123、-6.266,P值均<0.001;HBsAg:t值分别为-4.652、-4.691、-4.952、-4.804、-4.407,P值均<0.001)。 结论 基线高HBV DNA水平、HBsAg定量和低ALT水平的NAs初治CHB患者更易发生LLV,动态监测其变化对LLV的出现有重要意义。 Abstract:Objective To investigate the influencing factors for low-level viremia (LLV) and their dynamic changes in chronic hepatitis B (CHB) patients treated with nucleos(t)ide analogues (NAs) for the first time. Methods A retrospective analysis was performed for 78 CHB patients who attended Department of Infectious Diseases, The First Affiliated Hospital of Nanchang University, from November 2020 to March 2022 and received antiviral therapy with NAs for at least 12 months, and according to HBV DNA level during treatment, they were divided into sustained virologic response (SVR) group with 58 patients and LLV group with 20 patients. The independent samples t-test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the Fisher's exact test was used for comparison of categorical data between two groups. The multivariate Logistic regression analysis was used to investigate the independent influencing factors for LLV and establish a predictive model, and the receiver operating characteristic (ROC) curve was used to evaluate the predictive value of this model. The Kaplan-Meier method was used to analyze cumulative HBV DNA negative conversion rate, and the Log-rank test was used for comparison. The analysis of variance with repeated measures was used to analyze the differences in HBV DNA and HBsAg between the two groups or within each group at weeks 0, 12, 24, 36, and 48. Results Compare with the SVR group, the LLV group had significantly higher HBeAg positive rate (90.0% vs 48.3%, χ2=10.701, P=0.001), log(HBV DNA) value (7.26±1.46 vs 5.65±1.70, t=-4.178, P < 0.001), and log(HBsAg) value (4.53±0.86 vs 3.44±0.93, t=-4.813, P < 0.001) and significantly lower age [29 (26-34) vs 33 (30-43), Z=-2.751, P=0.009], alanine aminotransferase (ALT) [67.0 (54.0-122.0)U/L vs 111.0 (47.0-406.0)U/L, Z=-2.203, P=0.028], aspartate aminotransferase [43.5 (32.8-62.8) U/L vs 77.5 (35.0-213.0)U/L, Z=-2.466, P=0.014], and liver stiffness measurement [7.7 (6.3-8.5)kPa vs 8.9 (7.2-11.4)kPa, Z=-2.022, P=0.043]. The multivariate logistic regression analysis showed that baseline HBV DNA (odds ratio [OR]=2.365, 95% confidence interval [CI]: 1.220-4.587, P=0.011), HBsAg (OR=4.229, 95% CI: 1.098-16.287, P=0.036), and ALT (OR=0.965, 95% CI: 0.937-0.994, P=0.018) were independent influencing factors for LLV in CHB patients, and the predictive model of Logit(MLLV)=-8.668+1.441×lgHBsAg+0.598×lgHBV DNA-0.016×ALT was established based on these factors, which had a larger area under the ROC curve than HBV DNA, HBsAg, and ALT (0.931 vs 0.774/0.856/0.666), with a sensitivity of 85.00% and a specificity of 93.10% at the optimal cut-off value of 0.44. The CHB patients with baseline HBV DNA > 7.29 lgIU/mL or HBsAg > 4.38 lgIU/mL had a significantly lower DNA negative conversion rate than those with DNA ≤7.29 lgIU/mL or HBsAg ≤4.38 lgIU/mL (χ2=22.52 and 26.35, both P < 0.001). In the CHB patients, the highest reduction rates of HBV DNA and HBsAg were observed at weeks 12 and 24, respectively, and the LLV group had significantly higher levels of HBV DNA and HBsAg than the SVR group at weeks 0, 12, 24, 36, and 48 (HBV DNA: t=-4.084, -4.526, -5.688, -7.123, and -6.266, all P < 0.001; HBsAg: t=-4.652, -4.691, -4.952, -4.804, and -4.407, all P < 0.001). Conclusion For the CHB patients treated with NAs for the first time, those with high HBV DNA load, high HBsAg quantification, and low ALT level at baseline are more likely to develop LLV, and dynamic monitoring of these indices is of great significance to observe the onset of LLV. -
Key words:
- Hepatitis B, Chronic /
- Low-level Viremia /
- Forecasting
-
表 1 CHB患者基线临床特征和实验室指标分析
Table 1. Analysis of baseline clinical characteristics and laboratory indexes of CHB patients
项目 所有患者(n=78) SVR组(n=58) LLV组(n=20) 统计值 P值 年龄(岁) 32(29~39) 33(30~43) 29(26~34) Z=-2.751 0.009 男性[例(%)] 54(69.2) 40(68.9) 14(70.0) χ2=0.007 0.931 家族史[例(%)] 22(28.2) 16(27.6) 6(30.0) χ2=0.043 0.836 BMI(kg/m2) 23.3±3.7 23.7±4.0 22.3±2.5 t=1.359 0.164 HBeAg阳性[例(%)] 46(59.0) 28(48.3) 18(90.0) χ2=10.701 0.001 lgHBV DNA(IU/mL) 6.07±1.78 5.65±1.70 7.26±1.46 t=-4.178 <0.001 lgHBsAg(IU/mL) 3.72±1.02 3.44±0.93 4.53±0.86 t=-4.813 <0.001 抗-HBc 8.50(7.51~9.83) 8.56(7.65~9.80) 8.46(6.91~9.87) Z=-0.092 0.927 AST(U/L) 68.0(38.5~125.5) 77.5(35.0~213.0) 43.5(32.8~62.8) Z=-2.466 0.014 ALT(U/L) 105.0(53.4~218.0) 111.0(47.0~406.0) 67.0(54.0~122.0) Z=-2.203 0.028 TBil(μmol/L) 15.6(10.2~32.5) 17.0(11.2~36.6) 15.0(9.3~20.7) Z=-1.087 0.277 Alb(g/L) 44.5(40.2~46.9) 43.6(38.4~46.5) 46.3(42.7~48.5) Z=2.352 0.073 TG(mmol/L) 1.00(0.63~1.44) 1.03(0.62~1.57) 0.96(0.76~13.20) Z=-0.069 0.945 Cr(μmol/L) 65.6(56.7~76.4) 62.3(54.9~73.5) 68.2(56.1~71.9) Z=2.432 0.065 PLT(×109) 181.7±65.9 174.3±67.3 202.7±58.4 t=-1.769 0.067 LSM(kPa) 8.1(6.8~11.2) 8.9(7.2~11.4) 7.7(6.3~8.5) Z=-2.022 0.043 肝硬化[例(%)] 25(32.1) 20(34.5) 5(25.0) χ2=0.614 0.433 抗病毒药物类型[例(%)] χ2=1.345 0.246 ETV初治 66(84.6) 49(84.5) 17(85.0) TDF或TAF初治 12(15.4) 9(15.5) 3(15.0) MAFLD[例(%)] 17(21.8) 13(22.4) 4(20.0) χ2=0.051 0.822 表 2 MLLV模型的建立
Table 2. Establishment of the MLLV model
变量 B值 SE Wald OR 95%CI P值 lgHBV DNA 0.589 0.245 5.932 1.818 1.124~2.942 0.015 lgHBsAg 1.441 0.586 6.051 4.225 1.340~13.316 0.014 ALT -0.016 0.007 4.918 0.984 0.969~0.998 0.027 常数 -8.668 2.355 13.551 0.001 <0.001 表 3 HBV DNA、HBsAg、ALT和MLLV对LLV发生的预测价值
Table 3. Predictive value of HBV DNA, HBsAg, ALT and MLLV for the occurrence of LLV
指标 AUC 95%CI 截断值 敏感度 特异度 P值 HBV DNA 0.774 0.665~0.861 7.29 70.00% 82.76% <0.001 HBsAg 0.856 0.758~0.925 4.38 85.00% 87.93% <0.001 ALT 0.666 0.550~0.769 139.80 95.00% 44.83% 0.007 MLLV 0.931 0.850~0.976 0.44 85.00% 93.10% <0.001 表 4 CHB患者治疗期间HBV DNA和HBsAg动态变化
Table 4. Dynamic changes of HBV DNA level and HBsAg level in CHB patients during treatment
时间 SVR组(n=58) LLV组 (n=20) t值 P值 HBV DNA(lgIU/mL) 基线 5.62±1.69 7.35±1.44 -4.084 <0.001 12周 2.62±1.261) 4.08±1.221) -4.526 <0.001 24周 1.82±0.731) 2.99±0.861) -5.688 <0.001 36周 1.43±0.371) 2.73±0.791) -7.123 <0.001 48周 1.28±0.341) 2.19±0.651) -6.266 <0.001 HBsAg(lgIU/mL) 基线 3.44±0.92 4.53±0.86 -4.652 <0.001 12周 3.23±0.801) 4.22±0.871) -4.691 <0.001 24周 3.16±0.701) 4.11±0.831) -4.952 <0.001 36周 3.12±0.661) 3.98±0.771) -4.804 <0.001 48周 3.00±0.691) 3.81±0.771) -4.407 <0.001 注:与同组基线比较,1)P<0.05。 -
[1] HUTIN Y, NASRULLAH M, EASTERBROOK P, et al. Access to treatment for hepatitis B virus infection-worldwide, 2016[J]. MMWR Morb Mortal Wkly Rep, 2018, 67(28): 773-777. DOI: 10.15585/mmwr.mm6728a2. [2] Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. [3] Chinese Society of Infectious Disease, Chinese Society of Hepatology, Chinese Medical Association. The expert consensus on clinical cure (functional cure) of chronic hepatitis B[J]. J Clin Hepatol, 2019, 35(8): 1693-1701. DOI: 10.3969/j.issn.1001-5256.2019.08.008.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎临床治愈(功能性治愈)专家共识[J]. 临床肝胆病杂志, 2019, 35(8): 1693-1701. DOI: 10.3969/j.issn.1001-5256.2019.08.008. [4] KIM HJ, CHO YK, JEON WK, et al. Clinical characteristics of patients with chronic hepatitis B who developed genotypic resistance to entecavir: Real-life experience[J]. Clin Mol Hepatol, 2017, 23(4): 323-330. DOI: 10.3350/cmh.2017.0005. [5] SHIN SK, YIM HJ, KIM JH, et al. Partial virological response after 2 years of entecavir therapy increases the risk of hepatocellular carcinoma in patients with hepatitis B virus-associated cirrhosis[J]. Gut Liver, 2021, 15(3): 430-439. DOI: 10.5009/gnl20074. [6] SUN Y, WU X, ZHOU J, et al. Persistent low level of hepatitis B virus promotes fibrosis progression during therapy[J]. Clin Gastroenterol Hepatol, 2020, 18(11): 2582-2591. e6. DOI: 10.1016/j.cgh.2020.03.001. [7] MAK LY, HUANG Q, WONG DK, et al. Residual HBV DNA and pgRNA viraemia is associated with hepatocellular carcinoma in chronic hepatitis B patients on antiviral therapy[J]. J Gastroenterol, 2021, 56(5): 479-488. DOI: 10.1007/s00535-021-01780-5. [8] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [9] LU FM, FENG B, ZHENG SJ, et al. Current status of the research on low-level viremia in chronic hepatitis B patients receiving nucleos(t)ide analogues[J]. J Clin Hepatol, 2021, 37(6): 1268-1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007.鲁凤民, 封波, 郑素军, 等. 核苷(酸)类似物经治的慢性乙型肝炎患者低病毒血症的研究现状[J]. 临床肝胆病杂志, 2021, 37(6): 1268-1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007. [10] SHEN JY, HE R, DENG HM, et al. Clinical efficacy of tenofovir in the treatment of chronic hepatitis B[J]. Int J Virol, 2021, 28(2): 154-157. DOI: 10.3760/cma.j.issn.1673-4092.2021.02.015.沈金勇, 何然, 邓红梅, 等. 替诺福韦治疗慢性乙型肝炎患者临床疗效分析[J]. 国际病毒学杂志, 2021, 28(2): 154-157. DOI: 10.3760/cma.j.issn.1673-4092.2021.02.015. [11] LI H, XU WT, DENG BC, et al. Research progress in the functional treatment of chronic hepatitis B with nucleoside (acid) analogues and pegylated interferon[J]. Clin J Med Offic, 2022, 50(9): 890-893. DOI: 10.16680/j.1671-3826.2022.09.04.李卉, 许文涛, 邓宝成, 等. 核苷(酸)类似物联合聚乙二醇干扰素功能性治愈慢性乙型肝炎研究进展[J]. 临床军医杂志, 2022, 50(9): 890-893. DOI: 10.16680/j.1671-3826.2022.09.04. [12] OGAWA E, NOMURA H, NAKAMUTA M, et al. Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B[J]. Liver Int, 2020, 40(7): 1578-1589. DOI: 10.1111/liv.14482. [13] AGARWAL K, BRUNETTO M, SETO WK, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection[J]. J Hepatol, 2018, 68(4): 672-681. DOI: 10.1016/j.jhep.2017.11.039. [14] LEE SB, JEONG J, PARK JH, et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir[J]. Clin Mol Hepatol, 2020, 26(3): 364-375. DOI: 10.3350/cmh.2020.0012. [15] KIM JH, SINN DH, KANG W, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment[J]. Hepatology, 2017, 66(2): 335-343. DOI: 10.1002/hep.28916. [16] REVILL PA, CHISARI FV, BLOCK JM, et al. A global scientific strategy to cure hepatitis B[J]. Lancet Gastroenterol Hepatol, 2019, 4(7): 545-558. DOI: 10.1016/S2468-1253(19)30119-0. [17] WU IC, LAI CL, HAN SH, et al. Efficacy of entecavir in chronic hepatitis B patients with mildly elevated alanine aminotransferase and biopsy-proven histological damage[J]. Hepatology, 2010, 51(4): 1185-1189. DOI: 10.1002/hep.23424. [18] ZHANG Q, PENG H, LIU X, et al. Chronic hepatitis B infection with low level viremia correlates with the progression of the liver disease[J]. J Clin Transl Hepatol, 2021, 9(6): 850-859. DOI: 10.14218/JCTH.2021.00046. [19] BAO T, HU QG, YE J, et al. Value of HBsAg level in dynamic monitoring of disease progression in patients with chronic HBV infection[J]. J Clin Hepatol, 2017, 33(8): 1475-1478. DOI: 10.3969/j.issn.1001-5256.2017.08.012.鲍腾, 胡庆刚, 叶珺, 等. HBsAg水平在慢性HBV感染者疾病进展中的动态监测价值[J]. 临床肝胆病杂志, 2017, 33(8): 1475-1478. DOI: 10.3969/j.issn.1001-5256.2017.08.012. [20] SONG JC, MIN BY, KIM JW, et al. Pretreatment serum HBsAg-to-HBV DNA ratio predicts a virologic response to entecavir in chronic hepatitis B[J]. Korean J Hepatol, 2011, 17(4): 268-273. DOI: 10.3350/kjhep.2011.17.4.268. [21] LEE JM, AHN SH, KIM HS, et al. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir[J]. Hepatology, 2011, 53(5): 1486-1493. DOI: 10.1002/hep.24221. [22] CHEN H, FU JJ, LI L, et al. Influencing factors for low-level viremia in chronic hepatitis B patients treated with long-term entecavir antiviral therapy[J]. J Clin Hepatol, 2021, 37(3): 556-559. DOI: 10.3969/j.issn.1001-5256.2021.03.011.陈贺, 傅涓涓, 李丽, 等. 长期恩替卡韦经治慢性乙型肝炎患者低病毒血症的相关影响因素[J]. 临床肝胆病杂志, 2021, 37(3): 556-559. DOI: 10.3969/j.issn.1001-5256.2021.03.011. [23] LIM SG, PHYO WW, LING J, et al. Comparative biomarkers for HBsAg loss with antiviral therapy shows dominant influence of quantitative HBsAg (qHBsAg)[J]. Aliment Pharmacol Ther, 2021, 53(1): 172-182. DOI: 10.1111/apt.16149. 期刊类型引用(1)
1. 梁辰飞,程艳丽. 十二指肠旁胰腺炎1例报道并文献复习. 中国当代医药. 2022(28): 171-174 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2326 KB)
PDF下载 ( 2326 KB)

 下载:
下载:

 百度学术
百度学术

