缺氧诱导因子对肝细胞癌的作用机制及临床意义
DOI: 10.3969/j.issn.1001-5256.2022.03.040
Mechanism of action and clinical significance of hypoxia-inducible factors in hepatocellular carcinoma
-
摘要: 由于肿瘤细胞快速增殖生长,许多实体瘤中存在缺氧的微环境,缺氧诱导因子对于感知肿瘤内的氧张力并随后介导缺氧反应激活至关重要。肝细胞癌(HCC)是最缺氧的肿瘤之一,本文总结了缺氧诱导因子通过促进糖酵解、血管生成、侵袭转移、免疫逃避、癌症干细胞化等方式促进HCC发生发展的作用机制。当前,缺氧诱导因子抑制剂靶向药物的研发在HCC治疗领域具有广阔前景,缺氧诱导因子检测对HCC预后评估也具有潜在价值。Abstract: Due to the rapid proliferation and growth of tumor cells, hypoxic microenvironment exists in many solid tumors, and hypoxia inducible factors (HIF) are critical for sensing oxygen tension within tumors and subsequently mediating the activation of hypoxia responses. Hepatocellular carcinoma (HCC) is one of the most hypoxic tumors. This article summarizes the mechanism of action of HIF in promoting the development and progression of HCC by promoting glycolysis, angiogenesis, invasion and metastasis, immune escape, and cancer stem cell formation. At present, the development of targeted drugs for HIF inhibitors has broad prospects in the treatment of HCC, and the detection of HIF also has a potential value in prognostic evaluation HCC.
-
临床上,胰腺恶性肿瘤大部分为原发肿瘤,胰腺转移癌少见[1]。胰腺转移癌与胰腺原发肿瘤鉴别困难,极易造成误诊,延误治疗。目前,胰腺转移癌国内外尚无统一治疗标准,原发肿瘤的生物学特性和针对原发肿瘤的综合治疗是决定其预后的主要因素,个体化差异较大。近期,笔者接诊1例宫颈鳞癌胰腺转移患者,现将病例资料及经验总结报告如下。
1. 病例资料
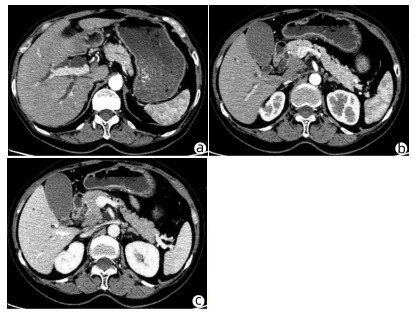
患者女性,63岁,因“皮肤、巩膜黄染20余天”于2021年3月27日入本院。起初黄染程度不重,后进行性加重,无腹痛腹胀,无阴道流血等其他不适。曾于2013年9月6日因阴道异常流血于外院行宫颈活检,病理学诊断为宫颈中分化鳞癌ⅢB期,予局部放疗联合TP方案(多西他赛+奥沙利铂)化疗,于2015年结束治疗后复查腹盆腔脏器、腹腔、盆腔均未见肿瘤转移。出院后患者再未复查,亦无特殊不适。入院查体:皮肤巩膜明显黄染,腹平坦,腹部无压痛,腹部未触及肿块。辅助检查:TBil 282.99 μmol/L,DBil 203.80 μmol/L,CA19-9正常;妇科彩超示:老年性子宫;全腹盆腔增强CT示:肝内外胆管扩张,主胰管可见(图 1a),胆囊明显增大,胆总管下段狭窄,动脉期见胰腺钩突区低密度肿块(图 1b),静脉期肿块轻度强化(图 1c),子宫显示不明显,腹膜后可见多发淋巴结肿大;磁共振胰胆管造影示:胰头区异常信号肿块,肝内外胆管扩张,胆汁淤积;超声内镜示:胰头钩突区占位,直径约3 cm。入院后予以护肝利胆治疗,患者黄疸进行性加重,建议患者行穿刺活检、PET-CT检查,患方拒绝。2021年4月4日行剖腹探查:术中探查发现肿瘤位于胰头钩突区,质地硬,侵犯十二指肠壁和胆总管,腹膜后见多发淋巴结肿大,行胰头十二指肠切除术。术后病理学检查结果:胰腺钩突肿块大小3.0 cm×2.0 cm×1.5 cm,癌组织侵犯十二指肠及胆总管,胰腺切缘未见癌。癌组织呈团块状或条索状癌巢(图 2a),癌细胞及核大小、形态不一,分布不规则,核分裂像多见,癌组织侵犯淋巴管及血管,脉管内见癌栓(图 2b),周围淋巴结可见癌转移(2/2)。免疫组化:P16(弥漫性+),P63(+),P40(+),CK7(+),CK8(+),CK19(+),ER(+)(图 2c、d)。诊断考虑胰腺钩突区中分化鳞状细胞癌,结合临床病史及免疫组化结果,考虑宫颈鳞癌转移。目前,该患者于本院进一步针对原发肿瘤行化疗等综合治疗,日常生活行为已恢复正常。
2. 讨论
据统计,宫颈癌的患病/病死率在女性恶性肿瘤中居第4位,鳞状细胞癌是最常见的病理类型,占比约80%,P16、P40等表达阳性对宫颈鳞癌诊断有重要意义。宫颈鳞癌早期往往无特殊表现,晚期可出现异常阴道流血、腰痛、盆腔痛、性交痛、贫血等表现,诊断时大多发生转移,多转移至直肠、膀胱、盆腔、肺、骨、肝等。临床上,胰腺癌多为原发肿瘤,胰腺转移癌罕见,仅占胰腺恶性肿瘤总数的2%~3%[2]。在一项纳入973例胰腺肿瘤手术标本病理资料的研究[3]中,共38例为胰腺转移瘤,主要包括淋巴瘤11例,胃癌7例,肾癌6例,肺癌2例,肝癌、前列腺癌、卵巢癌、子宫癌各1例,默克尔细胞癌1例,另有3例胃肠道恶性间质瘤和1例腹膜后平滑肌肉瘤;81例转移性胰腺肿瘤尸检报告显示,主要来源肺癌34例,胃肠道20例,肾脏4例,乳腺3例,肝脏2例,卵巢和膀胱各1例,另有6例来自造血系统,黑色素瘤、肉瘤和间皮瘤各2例。来源于宫颈的胰腺转移癌并不多见,笔者在国内外文献检索系统中,共检出各类型宫颈癌胰腺转移病例报告10例[4-7],其中鳞癌3例,神经内分泌癌5例,腺癌2例;转移灶位于胰头3例,位于颈部1例,位于胰腺体部3例,位于尾部、体尾部和胰头及体尾部各1例。转移灶位于胰头的3例患者中,1例为鳞癌,2例为神经内分泌癌,胰腺转移部位与宫颈癌病理类型无明显关联。本例患者在宫颈鳞癌同步放化疗后再未复查,6年后转移至胰腺钩突,临床表现为梗阻性黄疸,行胰头十二指肠切除术,术后病理学检查考虑鳞癌,结合免疫组化考虑为宫颈癌来源。目前,国内尚未见类似报道,国外也鲜有相关报道。
原发性胰腺癌与胰腺转移癌鉴别需依赖血清CA19-9水平、CT、MRI、磁共振胰胆管造影、内镜逆行胰胆管造影、超声内镜等检查手段。本例患者CT和MRI特点较原发性胰腺癌无特异表现,鉴别困难。血清CA19-9水平升高常见于胆道、胰腺恶性肿瘤,对诊断原发性胰腺癌有较高的敏感度和特异度[8]。鉴别困难时,可采用通过超声、CT及内镜引导下穿刺活检获取胰腺组织学、细胞学标本进行确诊,但需要评估穿刺活检的出血风险[9]。一般认为,超声内镜经十二指肠细针穿刺抽吸活检可提高胰腺疾病诊断的准确性,且相对安全[10-11]。本例患者术前影像学检查提示胰头钩突区占位,CA19-9水平不高,且有宫颈鳞癌病史,建议进一步行穿刺活检、PET-CT检查。但患者存在梗阻性黄疸,肝功能持续恶化,有手术指征,且患者家属不同意术前行穿刺活检、PET-CT等进一步检查,因此术前未能获得病理学诊断。
转移性胰腺癌临床表现因胰腺转移部位不同而异。在笔者检出的10例报告中,仅有1例(鳞癌)出现梗阻性黄疸,提示宫颈癌胰腺转移鲜有梗阻性黄疸症状,部分病例可因肿瘤压迫出现胆胰管扩张。转移性胰腺癌出现胰胆管梗阻较少见,可能与原发肿瘤主要经过淋巴及血行途径转移侵犯胰腺,不侵犯胆胰管有关[12]。本例患者胰腺转移性肿瘤致梗阻性黄疸,目前国内尚未见类似报道,国外仅有1例类似报道,但未分析相关转移机制。本例患者影像学检查示肝、胃、肠道等器官均未见肿瘤,但有腹膜后淋巴结肿大,病理学检查阳性;在既往10例报告中,3例胰腺转移性宫颈鳞癌有2例伴腹膜后淋巴结肿大,因此笔者推测宫颈鳞癌胰腺转移可能与腹膜后淋巴结侵犯相关。
关于转移性胰腺癌是否需要手术,意见尚未统一。以急腹症、进行性黄疸、出血为临床表现患者,应行急诊手术治疗,解除症状同时,切除病灶送检,根据病理学检查结果制订综合治疗方案。研究[13-14]表明,转移性胰腺癌行手术切除相较于保守治疗可延长生存期,且根治性手术相较于姑息性手术,预后更好。术后根据原发病灶辅以化疗等综合治疗,可提高治疗效果。对无法接受手术的转移性胰腺癌患者,应根据原发肿瘤的生物学特性制订以放疗为主的个体化综合治疗方案[15]。本例患者影像学检查提示存在胆道梗阻,并见胰头钩突区占位;胆红素水平持续升高,手术指征明确,手术切除胰腺钩突病灶、解除胆道梗阻后,胆红素水平明显下降,目前患者恢复良好,进一步接受化疗等综合治疗,延长生存期。
笔者经验总结:(1)对于诊断不明确的胰腺肿瘤,术前条件允许情况下需行穿刺活检明确性质,以指导下一步治疗方案;(2)胰腺转移癌在具备手术条件情况下,应积极采取以根治性手术为主的治疗方案,姑息性切除方案亦可行。胰腺转移癌为晚期癌症,术后需早期予以放化疗、靶向、免疫治疗等综合治疗;(3)对于不具备手术条件的患者,需根据原发肿瘤予以抗肿瘤综合治疗,包括放疗、化疗以及免疫治疗、靶向治疗等[16-17]。
-
[1] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. [2] GIGANTE E, PARADIS V, RONOT M, et al. New insights into the pathophysiology and clinical care of rare primary liver cancers[J]. JHEP Rep, 2021, 3(1): 100174. DOI: 10.1016/j.jhepr.2020.100174. [3] YUEN VW, WONG CC. Hypoxia-inducible factors and innate immunity in liver cancer[J]. J Clin Invest, 2020, 130(10): 5052-5062. DOI: 10.1172/JCI137553. [4] MÉNDEZ-BLANCO C, FERNÁNDEZ-PALANCA P, FONDEVILA F, et al. Prognostic and clinicopathological significance of hypoxia-inducible factors 1α and 2α in hepatocellular carcinoma: A systematic review with meta-analysis[J]. Ther Adv Med Oncol, 2021, 13: 1758835920987071. DOI: 10.1177/1758835920987071. [5] ZHANG Y, COLEMAN M, BREKKEN RA. Perspectives on hypoxia signaling in tumor stroma[J]. Cancers (Basel), 2021, 13(12): 3070. DOI: 10.3390/cancers13123070. [6] KOU HB, HAN DB, FAN YS, et al. Expression and clinical significance of hypoxia-inducible factor 1 α and E-cadherin in renal clear cell carcinoma[J]. Clin J Med Offic, 2020, 48(6): 649-652. DOI: 10.16680/j.1671-3826.2020.06.09.寇宏博, 韩冬冰, 范以生, 等. 缺氧诱导因子1α、上皮钙粘附蛋白在肾透明细胞癌中表达及临床意义[J]. 临床军医杂志, 2020, 48(6): 649-652. DOI: 10.16680/j.1671-3826.2020.06.09. [7] MYLONIS I, SIMOS G, PARASKEVA E. Hypoxia-inducible factors and the regulation of lipid metabolism[J]. Cells, 2019, 8(3): 214. DOI: 10.3390/cells8030214. [8] PASCALE RM, CALVISI DF, SIMILE MM, et al. The warburg effect 97 years after its discovery[J]. Cancers (Basel), 2020, 12(10): 2819. DOI: 10.3390/cancers12102819. [9] DONG F, LI R, WANG J, et al. Hypoxia-dependent expression of MAP17 coordinates the Warburg effect to tumor growth in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2021, 40(1): 121. DOI: 10.1186/s13046-021-01927-5. [10] PATEL MS, MAHMOOD S, JUNG J, et al. Reprogramming of aerobic glycolysis in non-transformed mouse liver with pyruvate dehydrogenase complex deficiency[J]. Physiol Rep, 2021, 9(1): e14684. DOI: 10.14814/phy2.14684. [11] ZHANG MY, ZHANG RJ, JIANG HJ, et al. 18F-fluoromisonidazole positron emission tomography may be applicable in the evaluation of colorectal cancer liver metastasis[J]. Hepatobiliary Pancreat Dis Int, 2019, 18(2): 164-172. DOI: 10.1016/j.hbpd.2019.02.008. [12] SONG HS, SONG YH, SINGH N, et al. New self-assembled supramolecular bowls as potent anticancer agents for human hepatocellular carcinoma[J]. Sci Rep, 2019, 9(1): 242. DOI: 10.1038/s41598-018-36755-9. [13] CHEN J, CHEN J, HUANG J, et al. HIF-2α upregulation mediated by hypoxia promotes NAFLD-HCC progression by activating lipid synthesis via the PI3K-AKT-mTOR pathway[J]. Aging (Albany NY), 2019, 11(23): 10839-10860. DOI: 10.18632/aging.102488. [14] JIANG XL, LI YC, JIANG XC, et al. HIF-mediated DEPTOR participates in the invasion and angiogenesis of liver cancer HepG2 cells through the PDCD4c-Jun(AP-1) signaling pathway[J]. J Clin Exp Med, 2020, 19(23): 2498-2501. DOI: 10.3969/j.issn.1671-4695.2020.23.010.蒋小丽, 李阳超, 蒋叙川, 等. HIF介导的DEPTOR通过PDCD4c-Jun(AP-1)信号通路参与肝癌HepG2细胞侵袭和血管生成研究[J]. 临床和实验医学杂志, 2020, 19(23): 2498-2501. DOI: 10.3969/j.issn.1671-4695.2020.23.010. [15] WU L, ZHOU J, ZHOU W, et al. Sorafenib blocks the activation of the HIF-2α/VEGFA/EphA2 pathway, and inhibits the rapid growth of residual liver cancer following high-intensity focused ultrasound therapy in vivo[J]. Pathol Res Pract, 2021, 220: 153270. DOI: 10.1016/j.prp.2020.153270. [16] YU J, SHI X, YANG C, et al. A novel germline gain-of-function HIF2A mutation in hepatocellular carcinoma with polycythemia[J]. Aging (Albany NY), 2020, 12(7): 5781-5791. DOI: 10.18632/aging.102967. [17] MARIOTTI V, FIOROTTO R, CADAMURO M, et al. New insights on the role of vascular endothelial growth factor in biliary pathophysiology[J]. JHEP Rep, 2021, 3(3): 100251. DOI: 10.1016/j.jhepr.2021.100251. [18] WEI X, ZHAO L, REN R, et al. MiR-125b loss activated HIF1α/pAKT loop, leading to transarterial chemoembolization resistance in hepatocellular carcinoma[J]. Hepatology, 2021, 73(4): 1381-1398. DOI: 10.1002/hep.31448. [19] YANG B, PAN CS, LI Q, et al. Inhibitory effects of Chanling Gao on the proliferation and liver metastasis of transplanted colorectal cancer in nude mice[J]. PLoS One, 2019, 14(2): e0201504. DOI: 10.1371/journal.pone.0201504. [20] HE Y, YANG W, GAN L, et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol, 2021, 44(5): 355-365. DOI: 10.1016/j.gastrohep.2020.09.014. [21] TIRPE AA, GULEI D, CIORTEA SM, et al. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes[J]. Int J Mol Sci, 2019, 20(24): 6140. DOI: 10.3390/ijms20246140. [22] GURZU S, KOBORI L, FODOR D, et al. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: A review[J]. Biomed Res Int, 2019, 2019: 2962580. DOI: 10.1155/2019/2962580. [23] KHALAF K, HANA D, CHOU JT, et al. Aspects of the tumor microenvironment involved in immune resistance and drug resistance[J]. Front Immunol, 2021, 12: 656364. DOI: 10.3389/fimmu.2021.656364. [24] TAKEMOTO R, KAMIYA T, ATOBE T, et al. Regulation of lysyl oxidase expression in THP-1 cell-derived M2-like macrophages[J]. J Cell Biochem, 2021, 122(8): 777-786. DOI: 10.1002/jcb.29911. [25] LIU Y, TAN J, OU S, et al. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis[J]. J Physiol Biochem, 2019, 75(3): 391-401. DOI: 10.1007/s13105-019-00692-6. [26] CHEN Y, HUANG F, DENG L, et al. HIF-1-miR-219-SMC4 regulatory pathway promoting proliferation and migration of HCC under hypoxic condition[J]. Biomed Res Int, 2019, 2019: 8983704. DOI: 10.1155/2019/8983704. [27] DOU C, ZHOU Z, XU Q, et al. Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2+/PI3K/AKT pathway[J]. Oncogene, 2019, 38(8): 1239-1255. DOI: 10.1038/s41388-018-0505-8. [28] SONG Y, JIN X, LIU Y, et al. Long noncoding RNA ZFPM2-AS1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by regulating the miR-576-3p/HIF-1α axis[J]. Anticancer Drugs, 2021, 32(8): 812-821. DOI: 10.1097/CAD.0000000000001070. [29] YE Y, PENG Y, LI Y, et al. Effect of lincRNA-p21 targeting HIF-1α on biological functions of liver cancer cells[J]. Oncol Lett, 2019, 17(6): 4964-4968. DOI: 10.3892/ol.2019.10195. [30] CUI C, FU K, YANG L, et al. Hypoxia-inducible gene 2 promotes the immune escape of hepatocellular carcinoma from nature killer cells through the interleukin-10-STAT3 signaling pathway[J]. J Exp Clin Cancer Res, 2019, 38(1): 229. DOI: 10.1186/s13046-019-1233-9. [31] PIÑEIRO FERNÁNDEZ J, LUDDY KA, HARMON C, et al. Hepatic tumor microenvironments and effects on NK cell phenotype and function[J]. Int J Mol Sci, 2019, 20(17): 4131. DOI: 10.3390/ijms20174131. [32] WU Q, ZHOU W, YIN S, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer[J]. Hepatology, 2019, 70(1): 198-214. DOI: 10.1002/hep.30593. [33] WEN Q, HAN T, WANG Z, et al. Role and mechanism of programmed death-ligand 1 in hypoxia-induced liver cancer immune escape[J]. Oncol Lett, 2020, 19(4): 2595-2601. DOI: 10.3892/ol.2020.11369. [34] HAJIZADEH F, OKOYE I, ESMAILY M, et al. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells[J]. Life Sci, 2019, 237: 116952. DOI: 10.1016/j.lfs.2019.116952. [35] SUN X, LV X, YAN Y, et al. Hypoxia-mediated cancer stem cell resistance and targeted therapy[J]. Biomed Pharmacother, 2020, 130: 110623. DOI: 10.1016/j.biopha.2020.110623. [36] AKANJI MA, ROTIMI D, ADEYEMI OS. Hypoxia-inducible factors as an alternative source of treatment strategy for cancer[J]. Oxid Med Cell Longev, 2019, 2019: 8547846. DOI: 10.1155/2019/8547846. [37] LI Y, GUO P, TIAN YY. Effects of Huangqin Decoction combined with sequential hepatic arterial chemoembolization on the expression of NF-κB and HIF-1α in patients with primary liver cancer[J]. China J Tradit Chin Med Pharma, 2019, 34(8): 3870-3873. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201908146.htm李岩, 郭鹏, 田月洋. 黄芩汤联合序贯肝动脉化疗栓塞术对原发性肝癌患者核因子κB及细胞缺氧诱导因子-1α的影响[J]. 中华中医药杂志, 2019, 34(8): 3870-3873. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201908146.htm [38] 张健, 彭勇, 马海, 等. 水飞蓟素对缺氧人肝癌HepG-2细胞HIF-1α及MDR1表达的影响[J/CD]. 肿瘤代谢与营养电子杂志, 2021, 8(2): 175-178. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLDX202102016.htm [39] LIANG C, DONG Z, CAI X, et al. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma[J]. Cell Death Dis, 2020, 11(11): 1017. DOI: 10.1038/s41419-020-03233-y. [40] FENG J, DAI W, MAO Y, et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis[J]. J Exp Clin Cancer Res, 2020, 39(1): 24. DOI: 10.1186/s13046-020-1528-x. [41] ZHAO JJ, ZHANG HG, CUI FF, et al. Effect of hypoxia-inducible factor-1α on stemness and epirubicin sensitivity of HepG2 hepatoma cells[J]. J Clin Hepatol, 2021, 37(2): 354-357. DOI: 10.3969/j.issn.1001-5256.2021.02.021.赵金金, 张海光, 崔非非, 等. 缺氧诱导因子1α对肝癌细胞HepG2干细胞特性及表阿霉素敏感性的影响[J]. 临床肝胆病杂志, 2021, 37(2): 354-357. DOI: 10.3969/j.issn.1001-5256.2021.02.021. [42] JU C, COLGAN SP, ELTZSCHIG HK. Hypoxia-inducible factors as molecular targets for liver diseases[J]. J Mol Med (Berl), 2016, 94(6): 613-627. DOI: 10.1007/s00109-016-1408-1. [43] DENG F, CHEN D, WEI X, et al. Development and validation of a prognostic classifier based on HIF-1 signaling for hepatocellular carcinoma[J]. Aging (Albany NY), 2020, 12(4): 3431-3450. DOI: 10.18632/aging.102820. [44] WANG D, LU S, ZHANG X, et al. Co-expression of KIAA1199 and hypoxia-inducible factor 1α is a biomarker for an unfavorable prognosis in hepatocellular carcinoma[J]. Medicine (Baltimore), 2020, 99(50): e23369. DOI: 10.1097/MD.0000000000023369. [45] LI Q, NI Y, ZHANG L, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation[J]. Signal Transduct Target Ther, 2021, 6(1): 76. DOI: 10.1038/s41392-020-00453-8. [46] FANG X, ZHANG D, ZHAO W, et al. Dishevelled associated activator of morphogenesis (DAAM) facilitates invasion of hepatocellular carcinoma by upregulating hypoxia-inducible factor 1α (HIF-1α) expression[J]. Med Sci Monit, 2020, 26: e924670. DOI: 10.12659/MSM.924670. [47] QIAN Y, LI Y, ZHENG C, et al. High methylation levels of histone H3 lysine 9 associated with activation of hypoxia-inducible factor 1α (HIF-1α) predict patients' worse prognosis in human hepatocellular carcinomas[J]. Cancer Genet, 2020, 245: 17-26. DOI: 10.1016/j.cancergen.2020.04.077. [48] JIANG Z, ZHOU Q, GE C, et al. Rpn10 promotes tumor progression by regulating hypoxia-inducible factor 1 alpha through the PTEN/Akt signaling pathway in hepatocellular carcinoma[J]. Cancer Lett, 2019, 447: 1-11. DOI: 10.1016/j.canlet.2019.01.020. [49] HIROTA K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs)[J]. Free Radic Biol Med, 2019, 133: 118-129. DOI: 10.1016/j.freeradbiomed.2018.07.018. [50] GUO Y, XIAO Z, YANG L, et al. Hypoxia?inducible factors in hepatocellular carcinoma (Review)[J]. Oncol Rep, 2020, 43(1): 3-15. DOI: 10.3892/or.2019.7397. [51] WANG P, YAN Q, LIAO B, et al. The HIF1α/HIF2α-miR210-3p network regulates glioblastoma cell proliferation, dedifferentiation and chemoresistance through EGF under hypoxic conditions[J]. Cell Death Dis, 2020, 11(11): 992. DOI: 10.1038/s41419-020-03150-0. 期刊类型引用(1)
1. 李帅,王娟,夏仕雪,顾鹏. 子宫颈混合性腺神经内分泌癌胰腺转移1例. 中国医学影像学杂志. 2022(12): 1283-1284 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2315 KB)
PDF下载 ( 2315 KB)


 下载:
下载:


 下载:
下载:
 百度学术
百度学术