新型冠状病毒感染抗病毒药物引起肝损伤的发生机制
DOI: 10.12449/JCH240230
Pathogenesis of liver injury caused by antiviral drugs for coronavirus disease 2019
-
摘要: 药物性肝损伤是药物使用过程中因药物本身和/或其代谢产物,或由于特殊体质对药物的超敏感性或耐受性降低所导致。在近三年抗击新型冠状病毒感染(COVID-19)的诊治过程中,抗病毒药物起到了非常重要的作用,但国内外关于抗COVID-19药物引起肝损伤的报道较多,且其导致肝损伤的发生机制尚未明确。本文对COVID-19抗病毒药物的种类及其引起肝损伤机制的相关研究进展作一综述,旨在促进抗病毒药物的合理使用。
-
关键词:
- 化学性与药物性肝损伤 /
- 新型冠状病毒 /
- 病理过程
Abstract: Drug-induced liver injury is caused by the drug itself and/or its metabolites during drug use or occurs due to hypersensitivity or reduced tolerance to the drug in a particular body type. In the last three years of the diagnosis and treatment of coronavirus disease 2019 (COVID-19), antiviral drugs have played a very important role, but there are many reports on liver injury caused by anti-COVID-19 drugs in China and globally, with unknown pathogenesis of liver injury caused by such drugs. This article reviews the research advances in the types of antiviral drugs for COVID-19 and their mechanism in inducing liver injury, in order to promote the rational use of antiviral drugs.-
Key words:
- Chemical and Drug Induced Liver Injury /
- SARS-CoV-2 /
- Pathologic Processes
-
临床上,胰腺恶性肿瘤大部分为原发肿瘤,胰腺转移癌少见[1]。胰腺转移癌与胰腺原发肿瘤鉴别困难,极易造成误诊,延误治疗。目前,胰腺转移癌国内外尚无统一治疗标准,原发肿瘤的生物学特性和针对原发肿瘤的综合治疗是决定其预后的主要因素,个体化差异较大。近期,笔者接诊1例宫颈鳞癌胰腺转移患者,现将病例资料及经验总结报告如下。
1. 病例资料
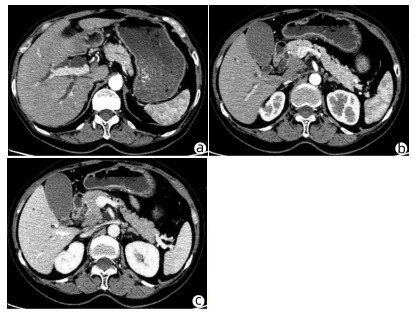
患者女性,63岁,因“皮肤、巩膜黄染20余天”于2021年3月27日入本院。起初黄染程度不重,后进行性加重,无腹痛腹胀,无阴道流血等其他不适。曾于2013年9月6日因阴道异常流血于外院行宫颈活检,病理学诊断为宫颈中分化鳞癌ⅢB期,予局部放疗联合TP方案(多西他赛+奥沙利铂)化疗,于2015年结束治疗后复查腹盆腔脏器、腹腔、盆腔均未见肿瘤转移。出院后患者再未复查,亦无特殊不适。入院查体:皮肤巩膜明显黄染,腹平坦,腹部无压痛,腹部未触及肿块。辅助检查:TBil 282.99 μmol/L,DBil 203.80 μmol/L,CA19-9正常;妇科彩超示:老年性子宫;全腹盆腔增强CT示:肝内外胆管扩张,主胰管可见(图 1a),胆囊明显增大,胆总管下段狭窄,动脉期见胰腺钩突区低密度肿块(图 1b),静脉期肿块轻度强化(图 1c),子宫显示不明显,腹膜后可见多发淋巴结肿大;磁共振胰胆管造影示:胰头区异常信号肿块,肝内外胆管扩张,胆汁淤积;超声内镜示:胰头钩突区占位,直径约3 cm。入院后予以护肝利胆治疗,患者黄疸进行性加重,建议患者行穿刺活检、PET-CT检查,患方拒绝。2021年4月4日行剖腹探查:术中探查发现肿瘤位于胰头钩突区,质地硬,侵犯十二指肠壁和胆总管,腹膜后见多发淋巴结肿大,行胰头十二指肠切除术。术后病理学检查结果:胰腺钩突肿块大小3.0 cm×2.0 cm×1.5 cm,癌组织侵犯十二指肠及胆总管,胰腺切缘未见癌。癌组织呈团块状或条索状癌巢(图 2a),癌细胞及核大小、形态不一,分布不规则,核分裂像多见,癌组织侵犯淋巴管及血管,脉管内见癌栓(图 2b),周围淋巴结可见癌转移(2/2)。免疫组化:P16(弥漫性+),P63(+),P40(+),CK7(+),CK8(+),CK19(+),ER(+)(图 2c、d)。诊断考虑胰腺钩突区中分化鳞状细胞癌,结合临床病史及免疫组化结果,考虑宫颈鳞癌转移。目前,该患者于本院进一步针对原发肿瘤行化疗等综合治疗,日常生活行为已恢复正常。
2. 讨论
据统计,宫颈癌的患病/病死率在女性恶性肿瘤中居第4位,鳞状细胞癌是最常见的病理类型,占比约80%,P16、P40等表达阳性对宫颈鳞癌诊断有重要意义。宫颈鳞癌早期往往无特殊表现,晚期可出现异常阴道流血、腰痛、盆腔痛、性交痛、贫血等表现,诊断时大多发生转移,多转移至直肠、膀胱、盆腔、肺、骨、肝等。临床上,胰腺癌多为原发肿瘤,胰腺转移癌罕见,仅占胰腺恶性肿瘤总数的2%~3%[2]。在一项纳入973例胰腺肿瘤手术标本病理资料的研究[3]中,共38例为胰腺转移瘤,主要包括淋巴瘤11例,胃癌7例,肾癌6例,肺癌2例,肝癌、前列腺癌、卵巢癌、子宫癌各1例,默克尔细胞癌1例,另有3例胃肠道恶性间质瘤和1例腹膜后平滑肌肉瘤;81例转移性胰腺肿瘤尸检报告显示,主要来源肺癌34例,胃肠道20例,肾脏4例,乳腺3例,肝脏2例,卵巢和膀胱各1例,另有6例来自造血系统,黑色素瘤、肉瘤和间皮瘤各2例。来源于宫颈的胰腺转移癌并不多见,笔者在国内外文献检索系统中,共检出各类型宫颈癌胰腺转移病例报告10例[4-7],其中鳞癌3例,神经内分泌癌5例,腺癌2例;转移灶位于胰头3例,位于颈部1例,位于胰腺体部3例,位于尾部、体尾部和胰头及体尾部各1例。转移灶位于胰头的3例患者中,1例为鳞癌,2例为神经内分泌癌,胰腺转移部位与宫颈癌病理类型无明显关联。本例患者在宫颈鳞癌同步放化疗后再未复查,6年后转移至胰腺钩突,临床表现为梗阻性黄疸,行胰头十二指肠切除术,术后病理学检查考虑鳞癌,结合免疫组化考虑为宫颈癌来源。目前,国内尚未见类似报道,国外也鲜有相关报道。
原发性胰腺癌与胰腺转移癌鉴别需依赖血清CA19-9水平、CT、MRI、磁共振胰胆管造影、内镜逆行胰胆管造影、超声内镜等检查手段。本例患者CT和MRI特点较原发性胰腺癌无特异表现,鉴别困难。血清CA19-9水平升高常见于胆道、胰腺恶性肿瘤,对诊断原发性胰腺癌有较高的敏感度和特异度[8]。鉴别困难时,可采用通过超声、CT及内镜引导下穿刺活检获取胰腺组织学、细胞学标本进行确诊,但需要评估穿刺活检的出血风险[9]。一般认为,超声内镜经十二指肠细针穿刺抽吸活检可提高胰腺疾病诊断的准确性,且相对安全[10-11]。本例患者术前影像学检查提示胰头钩突区占位,CA19-9水平不高,且有宫颈鳞癌病史,建议进一步行穿刺活检、PET-CT检查。但患者存在梗阻性黄疸,肝功能持续恶化,有手术指征,且患者家属不同意术前行穿刺活检、PET-CT等进一步检查,因此术前未能获得病理学诊断。
转移性胰腺癌临床表现因胰腺转移部位不同而异。在笔者检出的10例报告中,仅有1例(鳞癌)出现梗阻性黄疸,提示宫颈癌胰腺转移鲜有梗阻性黄疸症状,部分病例可因肿瘤压迫出现胆胰管扩张。转移性胰腺癌出现胰胆管梗阻较少见,可能与原发肿瘤主要经过淋巴及血行途径转移侵犯胰腺,不侵犯胆胰管有关[12]。本例患者胰腺转移性肿瘤致梗阻性黄疸,目前国内尚未见类似报道,国外仅有1例类似报道,但未分析相关转移机制。本例患者影像学检查示肝、胃、肠道等器官均未见肿瘤,但有腹膜后淋巴结肿大,病理学检查阳性;在既往10例报告中,3例胰腺转移性宫颈鳞癌有2例伴腹膜后淋巴结肿大,因此笔者推测宫颈鳞癌胰腺转移可能与腹膜后淋巴结侵犯相关。
关于转移性胰腺癌是否需要手术,意见尚未统一。以急腹症、进行性黄疸、出血为临床表现患者,应行急诊手术治疗,解除症状同时,切除病灶送检,根据病理学检查结果制订综合治疗方案。研究[13-14]表明,转移性胰腺癌行手术切除相较于保守治疗可延长生存期,且根治性手术相较于姑息性手术,预后更好。术后根据原发病灶辅以化疗等综合治疗,可提高治疗效果。对无法接受手术的转移性胰腺癌患者,应根据原发肿瘤的生物学特性制订以放疗为主的个体化综合治疗方案[15]。本例患者影像学检查提示存在胆道梗阻,并见胰头钩突区占位;胆红素水平持续升高,手术指征明确,手术切除胰腺钩突病灶、解除胆道梗阻后,胆红素水平明显下降,目前患者恢复良好,进一步接受化疗等综合治疗,延长生存期。
笔者经验总结:(1)对于诊断不明确的胰腺肿瘤,术前条件允许情况下需行穿刺活检明确性质,以指导下一步治疗方案;(2)胰腺转移癌在具备手术条件情况下,应积极采取以根治性手术为主的治疗方案,姑息性切除方案亦可行。胰腺转移癌为晚期癌症,术后需早期予以放化疗、靶向、免疫治疗等综合治疗;(3)对于不具备手术条件的患者,需根据原发肿瘤予以抗肿瘤综合治疗,包括放疗、化疗以及免疫治疗、靶向治疗等[16-17]。
-
[1] KAYAASLAN B, GUNER R. COVID-19 and the liver: A brief and core review[J]. World J Hepatol, 2021, 13( 12): 2013- 2023. DOI: 10.4254/wjh.v13.i12.2013. [2] CICHOŻ-LACH H, MICHALAK A. Liver injury in the era of COVID-19[J]. World J Gastroenterol, 2021, 27( 5): 377- 390. DOI: 10.3748/wjg.v27.i5.377. [3] CAI QX, HUANG DL, YU H, et al. COVID-19: Abnormal liver function tests[J]. J Hepatol, 2020, 73( 3): 566- 574. DOI: 10.1016/j.jhep.2020.04.006. [4] XIE YB, WANG SY, ZHANG C, et al. Research progress on the clinical features and mechanisms of COVID-19 combined with liver injury[J]. Chin J Hepatol, 2020, 28( 6): 523- 527. DOI: 10.3760/cma.j.cn501113-20200416-00190.谢云波, 王嗣予, 张超, 等. 新型冠状病毒肺炎合并肝损伤的临床特征及其机制研究进展[J]. 中华肝脏病杂志, 2020, 28( 6): 523- 527. DOI: 10.3760/cma.j.cn501113-20200416-00190. [5] TANG HL, ZHOU LY, LI XT, et al. Drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19: A disproportionality analysis of U.S. food and drug administration adverse event reporting system(FAERS) data[J]. Int J Clin Pharm, 2021, 43( 4): 1116- 1122. DOI: 10.1007/s11096-021-01311-5. [6] SAVIANO A, WRENSCH F, GHANY MG, et al. Liver disease and coronavirus disease 2019: From pathogenesis to clinical care[J]. Hepatology, 2021, 74( 2): 1088- 1100. DOI: 10.1002/hep.31684. [7] OLRY A, MEUNIER L, DÉLIRE B, et al. Drug-induced liver injury and COVID-19 infection: The rules remain the same[J]. Drug Saf, 2020, 43( 7): 615- 617. DOI: 10.1007/s40264-020-00954-z. [8] SAHA L, VIJ S, RAWAT K. Liver injury induced by COVID 19 treatment- what do we know?[J]. World J Gastroenterol, 2022, 28( 45): 6314- 6327. DOI: 10.3748/wjg.v28.i45.6314. [9] LEI F, LIU YM, ZHOU F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China[J]. Hepatology, 2020, 72( 2): 389- 398. DOI: 10.1002/hep.31301. [10] Chinese Medical Association, Journal of Chinese Medical Association, Gastroenterology Branch of Chinese Medical Association, et al. Guideline for primary care of drug-induced liver injury(2019)[J]. Chin J Gen Pract, 2020, 19( 10): 868- 875. DOI: 10.3760/cma.j.cn114798-20200812-00900.中华医学会, 中华医学会杂志社, 中华医学会消化病学分会, 等. 药物性肝损伤基层诊疗指南(2019年)[J]. 中华全科医师杂志, 2020, 19( 10): 868- 875. DOI: 10.3760/cma.j.cn114798-20200812-00900. [11] LAI RT, XIE Q. Clinical features of drug-induced liver failure and related diagnosis and treatment strategies[J]. J Clin Hepatol, 2021, 37( 11): 2534- 2538. DOI: 10.3969/j.issn.1001-5256.2021.11.006.赖荣陶, 谢青. 药物性肝衰竭的临床特点及诊治策略[J]. 临床肝胆病杂志, 2021, 37( 11): 2534- 2538. DOI: 10.3969/j.issn.1001-5256.2021.11.006. [12] ASHOUR NA, ELMAATY A ABO, SARHAN AA, et al. A systematic review of the global intervention for SARS-CoV-2 combating: From drugs repurposing to molnupiravir approval[J]. Drug Des Devel Ther, 2022, 16: 685- 715. DOI: 10.2147/DDDT.S354841. [13] Department of Health, Chinese Medicine Bureau. Notice on the issuance of the new coronavirus infection diagnosis and treatment program(Trial 10th edition)[Z]. 2023.中医药局综合司卫. 关于印发新型冠状病毒感染诊疗方案(试行第十版)的通知[Z]. 2023. [14] National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and reaseach information on drug-induced liver injury[EB/OL].[ 2023-11-10]. https. www.ncbi.nlm, nih. gov/books/. https [15] The State Food and Drug Administration approved the import registration of the combined packaging of Pfizer Covid-19 therapeutic drug Nimatvir tablets and Ritonavir tablets with emergency conditions[J]. China Health Nutrition, 2022, 32( 5): Insert 5.国家药监局应急附条件批准辉瑞公司新冠病毒治疗药物奈玛特韦片/利托那韦片组合包装进口注册[J]. 中国保健营养, 2022, 32( 5): 前插5. [16] KANG CK, SEONG MW, CHOI SJ, et al. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses[J]. Korean J Intern Med, 2020, 35( 4): 782- 787. DOI: 10.3904/kjim.2020.157. [17] ZAONGO SD, OUYANG J, CHEN YL, et al. HIV infection predisposes to increased chances of HBV infection: Current understanding of the mechanisms favoring HBV infection at each clinical stage of HIV infection[J]. Front Immunol, 2022, 13: 853346. DOI: 10.3389/fimmu.2022.853346. [18] KASPAR MB, STERLING RK. Mechanisms of liver disease in patients infected with HIV[J]. BMJ Open Gastroenterol, 2017, 4( 1): e000166. DOI: 10.1136/bmjgast-2017-000166. [19] VILLANUEVA-PAZ M, MORÁN L, LÓPEZ-ALCÁNTARA N, et al. Oxidative stress in drug-induced liver injury(DILI): From mechanisms to biomarkers for use in clinical practice[J]. Antioxidants(Basel), 2021, 10( 3): 390. DOI: 10.3390/antiox10030390. [20] LI XC, WANG WT, YAN SY, et al. Drug-induced liver injury in COVID-19 treatment: Incidence, mechanisms and clinical management[J]. Front Pharmacol, 2022, 13: 1019487. DOI: 10.3389/fphar.2022.1019487. [21] KAO E, SHINOHARA M, FENG M, et al. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes[J]. Hepatology, 2012, 56( 2): 594- 604. DOI: 10.1002/hep.25702. [22] ZHANG R, WU YN, HAN XX, et al. Research of anti-HIV drug-related liver injury[J]. Chin J Gastroenterol Hepatol, 2021, 30( 5): 490- 495. DOI: 10.3969/j.issn.1006-5709.2021.05.003.张瑞, 吴苑妮, 韩晓旭, 等. 抗艾滋病病毒药物相关性肝损伤的研究[J]. 胃肠病学和肝病学杂志, 2021, 30( 5): 490- 495. DOI: 10.3969/j.issn.1006-5709.2021.05.003. [23] HU DH, LIU LM, XIA HM. Study on the damage of human immunodeficiency virus protease inhibitor to human hepatocytes and the mechanism of intracellular calcium homeostasis regulation[J]. Chin J Infect Dis, 2014, 32( 9): 559- 561. DOI: 10.3760/cma.j.issn.1000-6680.2014.09.014.胡东辉, 刘黎明, 夏红梅. 人类免疫缺陷病毒蛋白酶抑制剂对人肝细胞损伤及细胞内钙离子稳态调节机制研究[J]. 中华传染病杂志, 2014, 32( 9): 559- 561. DOI: 10.3760/cma.j.issn.1000-6680.2014.09.014. [24] FOUFELLE F, FROMENTY B. Role of endoplasmic reticulum stress in drug-induced toxicity[J]. Pharmacol Res Perspect, 2016, 4( 1): e00211. DOI: 10.1002/prp2.211. [25] NÚÑEZ M. Hepatotoxicity of antiretrovirals: Incidence, mechanisms and management[J]. J Hepatol, 2006, 44( 1 Suppl): S132- S139. DOI: 10.1016/j.jhep.2005.11.027. [26] NAIDOO K, HASSAN-MOOSA R, MLOTSHWA P, et al. High rates of drug-induced liver injury in people living with HIV coinfected with tuberculosis(TB) irrespective of antiretroviral therapy timing during antituberculosis treatment: Results from the starting antiretroviral therapy at three points in TB trial[J]. Clin Infect Dis, 2020, 70( 12): 2675- 2682. DOI: 10.1093/cid/ciz732. [27] YU YC, CHEN CW. Pathogenesis of drug-induced liver injury: Current understanding and future needs[J]. J Clin Hepatol, 2021, 37( 11): 2515- 2524. DOI: 10.3969/j.issn.1001-5256.2021.11.003.于乐成, 陈成伟. 药物性肝损伤的发生机制: 当前认识和未来需求[J]. 临床肝胆病杂志, 2021, 37( 11): 2515- 2524. DOI: 10.3969/j.issn.1001-5256.2021.11.003. [28] BOECKMANS J, RODRIGUES RM, DEMUYSER T, et al. COVID-19 and drug-induced liver injury: A problem of plenty or a petty point?[J]. Arch Toxicol, 2020, 94( 4): 1367- 1369. DOI: 10.1007/s00204-020-02734-1. [29] DROŻDŻAL S, ROSIK J, LECHOWICZ K, et al. An update on drugs with therapeutic potential for SARS-CoV-2(COVID-19) treatment[J]. Drug Resist Updat, 2021, 59: 100794. DOI: 10.1016/j.drup.2021.100794. [30] WANG Z, LIU HS, WANG XY. Drug interaction and clinical medication guidance of nirmatrelvir/ritonavir tablets[J]. Clin Med J, 2023, 21( 1): 21- 26. DOI: 10.3969/j.issn.1672-3384.2023.01.004.王钊, 刘鹤松, 王秀云. 奈玛特韦片/利托那韦片药物相互作用与用药指导[J]. 临床药物治疗杂志, 2023, 21( 1): 21- 26. DOI: 10.3969/j.issn.1672-3384.2023.01.004. [31] PRUIJSSERS AJ, DENISON MR. Nucleoside analogues for the treatment of coronavirus infections[J]. Curr Opin Virol, 2019, 35: 57- 62. DOI: 10.1016/j.coviro.2019.04.002. [32] Expert group of“Expert consensus on the application of azvudine tablets in the treatment of novel coronavirus infection”. Expert consensus on the application of azvudine tablets in the treatment of novel coronavirus infection[J]. China Pharm, 2023, 32( 3): 1- 6. DOI: 10.3969/j.issn.1006-4931.2023.03.001.《阿兹夫定片在新型冠状病毒感染救治中应用的专家共识》专家组. 阿兹夫定片在新型冠状病毒感染救治中应用的专家共识[J]. 中国药业, 2023, 32( 3): 1- 6. DOI: 10.3969/j.issn.1006-4931.2023.03.001. [33] JIANG JD. Study on azvudine, a new anti-covid-19 drug[J]. Chin J Med Guide, 2022, 24( 10): 947- 948. DOI: 10.3969/j.issn.1009-0959.2022.10.001.蒋建东. 抗新冠病毒新药阿兹夫定研究[J]. 中国医药导刊, 2022, 24( 10): 947- 948. DOI: 10.3969/j.issn.1009-0959.2022.10.001. [34] National Institutes of Health. Coronavirus Disease 2019(COVID-19) treatment guidelines[EB/OL].( 2021-04-21)[ 2022-09-26]. https://www.covid19treatmentguidelines.nih.gov. https://www.covid19treatmentguidelines.nih.gov [35] AGRAWAL U, RAJU R, UDWADIA ZF. Favipiravir: A new and emerging antiviral option in COVID-19[J]. Med J Armed Forces India, 2020, 76( 4): 370- 376. DOI: 10.1016/j.mjafi.2020.08.004. [36] PILKINGTON V, PEPPERRELL T, HILL A. A review of the safety of favipiravir- a potential treatment in the COVID-19 pandemic?[J]. J Virus Erad, 2020, 6( 2): 45- 51. DOI: 10.1016/S2055-6640(20)30016-9. [37] LIANG Q, ZENG J, WU J, et al. Nucleoside reverse transcriptase inhibitors induced hepatocellular mitochondrial DNA lesions and compensatory enhancement of mitochondrial function and DNA repair[J]. Int J Antimicrob Agents, 2018, 51( 3): 385- 392. DOI: 10.1016/j.ijantimicag.2017.08.017. [38] ZHANG YL, WANG BS, LIANG Q, et al. Mitochondrial DNA D-loop AG/TC transition mutation in cortical neurons of mice after long-term exposure to nucleoside analogues[J]. J Neurovirol, 2015, 21( 5): 500- 507. DOI: 10.1007/s13365-015-0347-x. [39] LEE H, HANES J, JOHNSON KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase[J]. Biochemistry, 2003, 42( 50): 14711- 14719. DOI: 10.1021/bi035596s. [40] CAROTHERS C, BIRRER K, VO M. Acetylcysteine for the treatment of suspected remdesivir-associated acute liver failure in COVID-19: A case series[J]. Pharmacotherapy, 2020, 40( 11): 1166- 1171. DOI: 10.1002/phar.2464. [41] GERAGHTY RJ, ALIOTA MT, BONNAC LF. Broad-spectrum antiviral strategies and nucleoside analogues[J]. Viruses, 2021, 13( 4): 667. DOI: 10.3390/v13040667. [42] WANG BX, FISH EN. Global virus outbreaks: Interferons as 1st responders[J]. Semin Immunol, 2019, 43: 101300. DOI: 10.1016/j.smim.2019.101300. [43] YANG YZ, YIN JZ, ZHU WY. Research progress on the interaction between interferon and virus[J]. Chin J Exp Clin Virol, 2014, 28( 1): 79- 80, Cover 3. DOI: 10.3760/cma.j.issn.1003-9279.2014.01.027.杨蕴芝, 殷建忠, 朱武洋. 干扰素与病毒相互作用的研究进展[J]. 中华实验和临床病毒学杂志, 2014, 28( 1): 79- 80, 封 3. DOI: 10.3760/cma.j.issn.1003-9279.2014.01.027. [44] ZHANG RZ, WANG Q, YANG JS. Impact of liver functions by repurposed drugs for COVID-19 treatment[J]. J Clin Transl Hepatol, 2022, 10( 4): 748- 756. DOI: 10.14218/JCTH.2021.00368. [45] YANG P, XU KJ, KONG LM, et al. Liver injury caused by antiviral agents for COVID-19[J]. Chin J Clin Infect Dis, 2020, 13( 2): 102- 108. DOI: 10.3760/cma.j.issn.1674-2397.2020.02.004.阳平, 徐凯进, 孔丽敏, 等. 新型冠状病毒肺炎治疗中抗病毒药物引起的肝损伤[J]. 中华临床感染病杂志, 2020, 13( 2): 102- 108. DOI: 10.3760/cma.j.issn.1674-2397.2020.02.004. [46] WOOD DA, ALEEM A, DAVIS D. Providing access to monoclonal antibody treatment of coronavirus(COVID-19) patients in rural and underserved areas[EB/OL].( 2023-01-25)[ 2023-01-25]. https://www.ncbi.nlm.nih.gov/books/NBK574538. https://www.ncbi.nlm.nih.gov/books/NBK574538 [47] SHAN SS, WANG RK, ZHANG Q, et al. China’s first approved novel neutralizing antibody combination therapy against SARS-cov-2—BRII-196/BRII-198[J]. Chin J Med Guide, 2022, 24( 1): 2- 8. DOI: 10.3969/j.issn.1009-0959.2022.01.001.单思思, 王若珂, 张绮, 等. 安巴韦单抗注射液(BRII-196)及罗米司韦单抗注射液(BRII-198)——中国首个自主知识产权新冠病毒中和抗体联合治疗药物[J]. 中国医药导刊, 2022, 24( 1): 2- 8. DOI: 10.3969/j.issn.1009-0959.2022.01.001. [48] ALEEM A, VAQAR S. Monoclonal antibody therapy for high-risk coronavirus(COVID 19) patients with mild to moderate disease presentations[EB/OL].( 2023-02-05)[ 2023-02-05]. https://www.ncbi.nlm.nih.gov/books/NBK570603/ https://www.ncbi.nlm.nih.gov/books/NBK570603/ [49] CONTI P, PREGLIASCO FE, CALVISI V, et al. Monoclonal antibody therapy in COVID-19[J]. J Biol Regul Homeost Agents, 2021, 35( 2): 423- 427. DOI: 10.23812/Conti_Edit_35_2_1. [50] ALEEM A, OLAREWAJU O, POZUN A. Evaluating and referring patients for outpatient monoclonal antibody therapy for coronavirus(COVID-19) in the emergency department[EB/OL].( 2023-01-25)[ 2023-01-25]. https://www.ncbi.nlm.nih.gov/books/NBK574561/. https://www.ncbi.nlm.nih.gov/books/NBK574561/ 期刊类型引用(1)
1. 李帅,王娟,夏仕雪,顾鹏. 子宫颈混合性腺神经内分泌癌胰腺转移1例. 中国医学影像学杂志. 2022(12): 1283-1284 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 669 KB)
PDF下载 ( 669 KB)


 下载:
下载:

 百度学术
百度学术
 下载:
下载: