磁共振质子密度脂肪分数(MRI-PDFF)在肝脂肪定量中的应用
DOI: 10.12449/JCH240327
Application of magnetic resonance imaging-proton density fat fraction in liver fat quantification
-
摘要: 不同病因的慢性肝病均可出现肝脂肪变性。引起肝脂肪性病变的主要诱因包括慢性病毒性肝炎、胆汁淤积性肝病、酒精和非酒精性脂肪性肝病等。肝脂肪变性初期表现为单纯性脂肪性肝病,继而出现脂肪性肝炎、肝纤维化、肝硬化,甚至肝细胞癌。随着医学影像技术的发展,磁共振质子密度脂肪分数(MRI-PDFF)已在临床广泛应用于脂肪性肝病(FLD)的诊断。MRI-PDFF因具有精确性高及可重复性好的优点,逐步成为FLD无创诊断的“金标准”。本文对MRI-PDFF在肝脂肪定量中的临床应用及研究进展进行综述。Abstract: Hepatic steatosis can be observed in chronic liver diseases of different etiologies. The main predisposing factors for hepatic steatosis include chronic viral hepatitis, cholestatic liver disease, alcoholic liver disease, and nonalcoholic fatty liver disease. Simple fatty liver disease is the initial manifestation of hepatic steatosis, followed by steatohepatitis, liver fibrosis, liver cirrhosis, and even hepatocellular carcinoma. With the development of medical imaging technology, magnetic resonance imaging-proton density fat fraction (MRI-PDFF) has been widely used in the diagnosis of fatty liver disease (FLD) in clinical practice. MRI-PDFF is gradually becoming the gold standard for the noninvasive diagnosis of FLD due to its high accuracy and good repeatability. This article reviews the clinical application of MRI-PDFF in liver fat quantification and related research advances.
-
Key words:
- Fatty Liver /
- Magnetic Resonance Imaging /
- Proton Density Fat Fraction /
- Diagnosis
-
临床上,胰腺恶性肿瘤大部分为原发肿瘤,胰腺转移癌少见[1]。胰腺转移癌与胰腺原发肿瘤鉴别困难,极易造成误诊,延误治疗。目前,胰腺转移癌国内外尚无统一治疗标准,原发肿瘤的生物学特性和针对原发肿瘤的综合治疗是决定其预后的主要因素,个体化差异较大。近期,笔者接诊1例宫颈鳞癌胰腺转移患者,现将病例资料及经验总结报告如下。
1. 病例资料
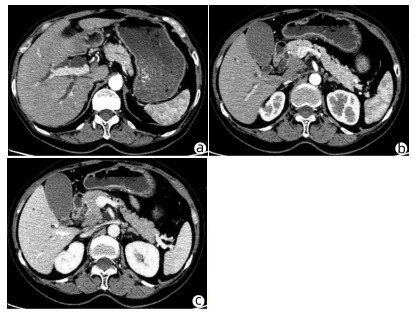
患者女性,63岁,因“皮肤、巩膜黄染20余天”于2021年3月27日入本院。起初黄染程度不重,后进行性加重,无腹痛腹胀,无阴道流血等其他不适。曾于2013年9月6日因阴道异常流血于外院行宫颈活检,病理学诊断为宫颈中分化鳞癌ⅢB期,予局部放疗联合TP方案(多西他赛+奥沙利铂)化疗,于2015年结束治疗后复查腹盆腔脏器、腹腔、盆腔均未见肿瘤转移。出院后患者再未复查,亦无特殊不适。入院查体:皮肤巩膜明显黄染,腹平坦,腹部无压痛,腹部未触及肿块。辅助检查:TBil 282.99 μmol/L,DBil 203.80 μmol/L,CA19-9正常;妇科彩超示:老年性子宫;全腹盆腔增强CT示:肝内外胆管扩张,主胰管可见(图 1a),胆囊明显增大,胆总管下段狭窄,动脉期见胰腺钩突区低密度肿块(图 1b),静脉期肿块轻度强化(图 1c),子宫显示不明显,腹膜后可见多发淋巴结肿大;磁共振胰胆管造影示:胰头区异常信号肿块,肝内外胆管扩张,胆汁淤积;超声内镜示:胰头钩突区占位,直径约3 cm。入院后予以护肝利胆治疗,患者黄疸进行性加重,建议患者行穿刺活检、PET-CT检查,患方拒绝。2021年4月4日行剖腹探查:术中探查发现肿瘤位于胰头钩突区,质地硬,侵犯十二指肠壁和胆总管,腹膜后见多发淋巴结肿大,行胰头十二指肠切除术。术后病理学检查结果:胰腺钩突肿块大小3.0 cm×2.0 cm×1.5 cm,癌组织侵犯十二指肠及胆总管,胰腺切缘未见癌。癌组织呈团块状或条索状癌巢(图 2a),癌细胞及核大小、形态不一,分布不规则,核分裂像多见,癌组织侵犯淋巴管及血管,脉管内见癌栓(图 2b),周围淋巴结可见癌转移(2/2)。免疫组化:P16(弥漫性+),P63(+),P40(+),CK7(+),CK8(+),CK19(+),ER(+)(图 2c、d)。诊断考虑胰腺钩突区中分化鳞状细胞癌,结合临床病史及免疫组化结果,考虑宫颈鳞癌转移。目前,该患者于本院进一步针对原发肿瘤行化疗等综合治疗,日常生活行为已恢复正常。
2. 讨论
据统计,宫颈癌的患病/病死率在女性恶性肿瘤中居第4位,鳞状细胞癌是最常见的病理类型,占比约80%,P16、P40等表达阳性对宫颈鳞癌诊断有重要意义。宫颈鳞癌早期往往无特殊表现,晚期可出现异常阴道流血、腰痛、盆腔痛、性交痛、贫血等表现,诊断时大多发生转移,多转移至直肠、膀胱、盆腔、肺、骨、肝等。临床上,胰腺癌多为原发肿瘤,胰腺转移癌罕见,仅占胰腺恶性肿瘤总数的2%~3%[2]。在一项纳入973例胰腺肿瘤手术标本病理资料的研究[3]中,共38例为胰腺转移瘤,主要包括淋巴瘤11例,胃癌7例,肾癌6例,肺癌2例,肝癌、前列腺癌、卵巢癌、子宫癌各1例,默克尔细胞癌1例,另有3例胃肠道恶性间质瘤和1例腹膜后平滑肌肉瘤;81例转移性胰腺肿瘤尸检报告显示,主要来源肺癌34例,胃肠道20例,肾脏4例,乳腺3例,肝脏2例,卵巢和膀胱各1例,另有6例来自造血系统,黑色素瘤、肉瘤和间皮瘤各2例。来源于宫颈的胰腺转移癌并不多见,笔者在国内外文献检索系统中,共检出各类型宫颈癌胰腺转移病例报告10例[4-7],其中鳞癌3例,神经内分泌癌5例,腺癌2例;转移灶位于胰头3例,位于颈部1例,位于胰腺体部3例,位于尾部、体尾部和胰头及体尾部各1例。转移灶位于胰头的3例患者中,1例为鳞癌,2例为神经内分泌癌,胰腺转移部位与宫颈癌病理类型无明显关联。本例患者在宫颈鳞癌同步放化疗后再未复查,6年后转移至胰腺钩突,临床表现为梗阻性黄疸,行胰头十二指肠切除术,术后病理学检查考虑鳞癌,结合免疫组化考虑为宫颈癌来源。目前,国内尚未见类似报道,国外也鲜有相关报道。
原发性胰腺癌与胰腺转移癌鉴别需依赖血清CA19-9水平、CT、MRI、磁共振胰胆管造影、内镜逆行胰胆管造影、超声内镜等检查手段。本例患者CT和MRI特点较原发性胰腺癌无特异表现,鉴别困难。血清CA19-9水平升高常见于胆道、胰腺恶性肿瘤,对诊断原发性胰腺癌有较高的敏感度和特异度[8]。鉴别困难时,可采用通过超声、CT及内镜引导下穿刺活检获取胰腺组织学、细胞学标本进行确诊,但需要评估穿刺活检的出血风险[9]。一般认为,超声内镜经十二指肠细针穿刺抽吸活检可提高胰腺疾病诊断的准确性,且相对安全[10-11]。本例患者术前影像学检查提示胰头钩突区占位,CA19-9水平不高,且有宫颈鳞癌病史,建议进一步行穿刺活检、PET-CT检查。但患者存在梗阻性黄疸,肝功能持续恶化,有手术指征,且患者家属不同意术前行穿刺活检、PET-CT等进一步检查,因此术前未能获得病理学诊断。
转移性胰腺癌临床表现因胰腺转移部位不同而异。在笔者检出的10例报告中,仅有1例(鳞癌)出现梗阻性黄疸,提示宫颈癌胰腺转移鲜有梗阻性黄疸症状,部分病例可因肿瘤压迫出现胆胰管扩张。转移性胰腺癌出现胰胆管梗阻较少见,可能与原发肿瘤主要经过淋巴及血行途径转移侵犯胰腺,不侵犯胆胰管有关[12]。本例患者胰腺转移性肿瘤致梗阻性黄疸,目前国内尚未见类似报道,国外仅有1例类似报道,但未分析相关转移机制。本例患者影像学检查示肝、胃、肠道等器官均未见肿瘤,但有腹膜后淋巴结肿大,病理学检查阳性;在既往10例报告中,3例胰腺转移性宫颈鳞癌有2例伴腹膜后淋巴结肿大,因此笔者推测宫颈鳞癌胰腺转移可能与腹膜后淋巴结侵犯相关。
关于转移性胰腺癌是否需要手术,意见尚未统一。以急腹症、进行性黄疸、出血为临床表现患者,应行急诊手术治疗,解除症状同时,切除病灶送检,根据病理学检查结果制订综合治疗方案。研究[13-14]表明,转移性胰腺癌行手术切除相较于保守治疗可延长生存期,且根治性手术相较于姑息性手术,预后更好。术后根据原发病灶辅以化疗等综合治疗,可提高治疗效果。对无法接受手术的转移性胰腺癌患者,应根据原发肿瘤的生物学特性制订以放疗为主的个体化综合治疗方案[15]。本例患者影像学检查提示存在胆道梗阻,并见胰头钩突区占位;胆红素水平持续升高,手术指征明确,手术切除胰腺钩突病灶、解除胆道梗阻后,胆红素水平明显下降,目前患者恢复良好,进一步接受化疗等综合治疗,延长生存期。
笔者经验总结:(1)对于诊断不明确的胰腺肿瘤,术前条件允许情况下需行穿刺活检明确性质,以指导下一步治疗方案;(2)胰腺转移癌在具备手术条件情况下,应积极采取以根治性手术为主的治疗方案,姑息性切除方案亦可行。胰腺转移癌为晚期癌症,术后需早期予以放化疗、靶向、免疫治疗等综合治疗;(3)对于不具备手术条件的患者,需根据原发肿瘤予以抗肿瘤综合治疗,包括放疗、化疗以及免疫治疗、靶向治疗等[16-17]。
-
[1] POWELL EE, JONSSON JR, CLOUSTON AD. Steatosis: co-factor in other liver diseases[J]. Hepatology, 2005, 42( 1): 5- 13. DOI: 10.1002/hep.20750. [2] TAMAKI N, AJMERA V, LOOMBA R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD[J]. Nat Rev Endocrinol, 2022, 18( 1): 55- 66. DOI: 10.1038/s41574-021-00584-0. [3] RATZIU V, CHARLOTTE F, HEURTIER A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease[J]. Gastroenterology, 2005, 128( 7): 1898- 1906. DOI: 10.1053/j.gastro.2005.03.084. [4] BOHTE AE, van WERVEN JR, BIPAT S, et al. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis[J]. Eur Radiol, 2011, 21( 1): 87- 97. DOI: 10.1007/s00330-010-1905-5. [5] JOHNSTON RJ, STAMM ER, LEWIN JM, et al. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: Limitations of liver-minus-spleen attenuation difference measurements[J]. Abdom Imaging, 1998, 23( 4): 409- 415. DOI: 10.1007/s002619900370. [6] FISCHER MA, GNANNT R, RAPTIS D, et al. Quantification of liver fat in the presence of iron and iodine: an ex-vivo dual-energy CT study[J]. Invest Radiol, 2011, 46( 6): 351- 358. DOI: 10.1097/RLI.0b013e31820e1486. [7] CHO YS, LIM S, KIM Y, et al. Abdominal wall thickness affects liver stiffness measurements by 2-D shear wave elastography in patients with chronic liver disease[J]. Ultrasound Med Biol, 2019, 45( 10): 2697- 2703. DOI: 10.1016/j.ultrasmedbio.2019.06.415. [8] MU R, LU LG. Advances in the application of magnetic resonance imaging in hepatic fat quantification in patients with nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36( 6): 1366- 1369. DOI: 10.3969/j.issn.1001-5256.2020.06.038.穆容, 陆伦根. 磁共振成像技术在非酒精性脂肪性肝病肝脂肪定量中的应用[J]. 临床肝胆病杂志, 2020, 36( 6): 1366- 1369. DOI: 10.3969/j.issn.1001-5256.2020.06.038. [9] European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. Obes Facts, 2016, 9( 2): 65- 90. DOI: 10.1159/000443344. [10] STERN C, CASTERA L. Non-invasive diagnosis of hepatic steatosis[J]. Hepatol Int, 2017, 11( 1): 70- 78. DOI: 10.1007/s12072-016-9772-z. [11] RUNGE JH, SMITS LP, VERHEIJ J, et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis[J]. Radiology, 2017: 162931. DOI: 10. 1148/radiol. 2017162931. [12] GUO D, YANG C. Progress in MRI research on fatty liver[J]. Med J West China, 2017, 29( 4): 419- 422.郭达, 杨陈. 脂肪肝的MRI研究进展[J]. 西部医学, 2017, 29( 4): 419- 422. [13] CAUSSY C, REEDER SB, SIRLIN CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials[J]. Hepatology, 2018, 68( 2): 763- 772. DOI: 10.1002/hep.29797. [14] BANNAS P, KRAMER H, HERNANDO D, et al. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers[J]. Hepatology, 2015, 62( 5): 1444- 1455. DOI: 10.1002/hep.28012. [15] REEDER SB, HU HH, SIRLIN CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration[J]. J Magn Reson Imaging, 2012, 36( 5): 1011- 1014. DOI: 10.1002/jmri.23741. [16] MAMIDIPALLI A, FOWLER KJ, HAMILTON G, et al. Prospective comparison of longitudinal change in hepatic proton density fat fraction(PDFF) estimated by magnitude-based MRI(MRI-M) and complex-based MRI(MRI-C)[J]. Eur Radiol, 2020, 30( 9): 5120- 5129. DOI: 10.1007/s00330-020-06858-x. [17] HERNANDO D, SHARMA SD, ALIYARI GHASABEH M, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom[J]. Magn Reson Med, 2017, 77( 4): 1516- 1524. DOI: 10.1002/mrm.26228. [18] YOSHIZAWA E, YAMADA A. MRI-derived proton density fat fraction[J]. J Med Ultrason(2001), 2021, 48( 4): 497- 506. DOI: 10.1007/s10396-021-01135-w. [19] VU KN, GILBERT G, CHALUT M, et al. MRI-determined liver proton density fat fraction, with MRS validation: Comparison of regions of interest sampling methods in patients with type 2 diabetes[J]. J Magn Reson Imaging, 2016, 43( 5): 1090- 1099. DOI: 10.1002/jmri.25083. [20] SATKUNASINGHAM J, HOSSEINI NIK H, Fischer S, et al. Can negligible hepatic steatosis determined by MRI-proton density fat fraction obviate the need for liver biopsy in potential liver donors?[J]. Liver Transpl, 2018, 24( 4): 470- 477. DOI: 10. 1002/lt. 24965. [21] CAMPO CA, HERNANDO D, SCHUBERT T, et al. Standardized approach for roi-based measurements of proton density fat fraction and R2* in the liver[J]. AJR Am J Roentgenol, 2017, 209( 3): 592- 603. DOI: 10.2214/AJR.17.17812. [22] LU WT, SHI HL, CHEN QL, et al. Distribution characteristics of MRI-PDFF in patients with metabolic related fatty liver disease[J/CD]. Chin J Clinicians: Electronic Edition, 2021, 15( 7): 503- 508. DOI: 10.3877/cma.j.issn.1674-0785.2021.07.005.陆玮婷, 史会连, 陈沁磊, 等. 代谢相关脂肪性肝病患者MRI-PDFF分布特点[J/CD]. 中华临床医师杂志: 电子版, 2021, 15( 7): 503- 508. DOI: 10.3877/cma.j.issn.1674-0785.2021.07.005. [23] PERMUTT Z, LE TA, PETERSON MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease- MRI accurately quantifies hepatic steatosis in NAFLD[J]. Aliment Pharmacol Ther, 2012, 36( 1): 22- 29. DOI: 10.1111/j.1365-2036.2012.05121.x. [24] SHAO CX, YE J, DONG Z, et al. Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease[J]. Ther Adv Chronic Dis, 2021, 12: 20406223211033119. DOI: 10.1177/20406223211033119. [25] TAMAKI N, MUNAGANURU N, JUNG J, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease[J]. Gut, 2022, 71( 5): 983- 990. DOI: 10.1136/gutjnl-2021-324264. [26] HARRISON SA, BASHIR MR, GUY CD, et al. Resmetirom(MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial[J]. Lancet, 2019, 394( 10213): 2012- 2024. DOI: 10.1016/S0140-6736(19)32517-6. [27] PARK CC, NGUYEN P, HERNANDEZ C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease[J]. Gastroenterology, 2017, 152( 3): 598- 607. e 2. DOI: 10.1053/j.gastro.2016.10.026. [28] LEE SJ, KIM SU. Noninvasive monitoring of hepatic steatosis: controlled attenuation parameter and magnetic resonance imaging-proton density fat fraction in patients with nonalcoholic fatty liver disease[J]. Expert Rev Gastroenterol Hepatol, 2019, 13( 6): 523- 530. DOI: 10.1080/17474124.2019.1608820. [29] JAYAKUMAR S, MIDDLETON MS, LAWITZ EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib[J]. J Hepatol, 2019, 70( 1): 133- 141. DOI: 10.1016/j.jhep.2018.09.024. [30] KIM HJ, CHO HJ, KIM B, et al. Accuracy and precision of proton density fat fraction measurement across field strengths and scan intervals: A phantom and human study[J]. J Magn Reson Imaging, 2019, 50( 1): 305- 314. DOI: 10.1002/jmri.26575. [31] QI Q, WEINSTOCK AK, CHUPETLOVSK AK, et al. Magnetic resonance imaging-derived proton density fat fraction(MRI-PDFF) is a viable alternative to liver biopsy for steatosis quantification in living liver donor transplantation[J]. Clin Transplant, 2021, 35( 7): e14339. DOI: 10.1111/ctr.14339. [32] REN H, YANG ZH. Progress in the application of MRI diagnostic techniques related to non-alcoholic fatty liver disease[J]. Radiol Pract, 2020, 35( 7): 928- 932. DOI: 10.13609/j.cnki.1000-0313.2020.07.019.任浩, 杨正汉. 非酒精性脂肪肝病相关MRI诊断技术的应用进展[J]. 放射学实践, 2020, 35( 7): 928- 932. DOI: 10.13609/j.cnki.1000-0313.2020.07.019. [33] MARTÍ-AGUADO D, ALBERICH-BAYARRI Á, MARTÍN-RODRÍGUEZ JL, et al. Differences in multi-echo chemical shift encoded MRI proton density fat fraction estimation based on multifrequency fat peaks selection in non-alcoholic fatty liver disease patients[J]. Clin Radiol, 2020, 75( 11): 880. e5- 880. e 12. DOI: 10.1016/j.crad.2020.07.031. [34] KIM JW, LEE CH, YANG Z, et al. The spectrum of magnetic resonance imaging proton density fat fraction(MRI-PDFF), magnetic resonance spectroscopy(MRS), and two different histopathologic methods(artificial intelligence vs. pathologist) in quantifying hepatic steatosis[J]. Quant Imaging Med Surg, 2022, 12( 11): 5251- 5262. DOI: 10.21037/qims-22-393. [35] IDILMAN IS, KESKIN O, CELIK A, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease[J]. Acta Radiol, 2016, 57( 3): 271- 278. DOI: 10.1177/0284185115580488. [36] YOKOO T, SERAI S D, PIRASTEH A, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using mr imaging: a meta-analysis[J]. Radiology, 2018, 286( 2): 486- 498. DOI: 10. 1148/radiol. 2017170550. [37] CHEN G, JIANG J, WANG X, et al. Evaluation of hepatic steatosis before liver transplantation in ex vivo by volumetric quantitative PDFF-MRI[J]. Magn Reson Med, 2021, 85( 5): 2805- 2814. DOI: 10.1002/mrm.28592. [38] GU J, LIU S, DU S, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis[J]. Eur Radiol, 2019, 29( 7): 3564- 3573. DOI: 10.1007/s00330-019-06072-4. [39] YANG YM, LIU YP, ZHOU DJ, et, al. The value of 3.0T MRI PDFF and IP-OP in quantitative evaluation of fatty liver[J]. J Jilin Univ(Med Edit), 2020, 46( 4): 875- 880. DOI: 10.13481/j.1671-587x.20200434.杨逸铭, 刘玉品, 周懂晶, 等. 3.0T MRI PDFF和IP-OP对脂肪肝定量评估的价值[J]. 吉林大学学报: 医学版, 2020, 46( 4): 875- 880. DOI: 10.13481/j.1671-587x.20200434. [40] STINE JG, MUNAGANURU N, BARNARD A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2021, 19( 11): 2274- 2283. e 5. DOI: 10.1016/j.cgh.2020.08.061. [41] LU JN, LI YS, WEN Y, et al. Application value of liver/spleen CT value, controlled attenuation parameter,and magnetic resonance imagingproton density fat fraction in chronic hepatitis B patients with hepatic steatosis[J]. J Clin Hepatol, 2024, 40( 1): 46- 51. DOI: 10.12449/JCH240109.鲁景楠, 李岩松, 温雅, 等. 肝/脾CT值、CAP和MRI-PDFF在慢性乙型肝炎脂肪变性患者中的应用价值[J]. 临床肝胆病杂志, 2024, 40( 1): 46- 51. DOI: 10.12449/JCH240109. [42] YANG YM, LIU YP, HUANG LX, et al. Quantitative evaluation of liver fat content in patients with chronic hepatitis B using magnetic resonance proton density fat fraction and transient elastography[J]. J Clin Hepatol, 2021, 37( 12): 2793- 2797. DOI: 10.3969/j.issn.1001-5256.2021.12.013.杨逸铭, 刘玉品, 黄丽霞, 等. 磁共振质子密度脂肪分数和瞬时弹性成像对慢性乙型肝炎患者肝脂肪含量的定量评估价值[J]. 临床肝胆病杂志, 2021, 37( 12): 2793- 2797. DOI: 10.3969/j.issn.1001-5256.2021.12.013. [43] CHO Y, RHEE H, KIM YE, et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial(ESSENTIAL study)[J]. BMC Med, 2022, 20( 1): 93. DOI: 10.1186/s12916-022-02288-2. [44] LU WT, LIU LN, SHI HL, et al. Clinical study on the treatment of 68 cases of non alcoholic fatty liver disease of liver depression and spleen deficiency type with“Jiuwei Qingzhi Cream”[J]. Jiangsu J Traditi Chin Med, 2022, 54( 7): 33- 36. DOI: 10.19844/j.cnki.1672-397X.陆玮婷, 刘丽娜, 史会连, 等.“九味清脂膏”治疗肝郁脾虚型非酒精性脂肪性肝病68例临床研究[J]. 江苏中医药, 2022, 54( 7): 33- 36. DOI: 10.19844/j.cnki.1672-397X. [45] ALKHOURI N, HERRING R, KABLER H, et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial[J]. J Hepatol, 2022, 77( 3): 607- 618. DOI: 10.1016/j.jhep.2022.04.003. [46] AN ZM, FENG Q. Magnetic resonance imaging-proton density fat fraction: A potential surrogate endpoint for nonalcoholic steatohepatitis clinical trials[J]. J Clin Hepatol, 2021, 37( 6): 1445- 1448. DOI: 10.3969/j.issn.1001-5256.2021.06.047.安梓铭, 冯琴. 磁共振质子密度脂肪分数——一种具有潜力的非酒精性脂肪性肝炎临床试验替代终点[J]. 临床肝胆病杂志, 2021, 37( 6): 1445- 1448. DOI: 10.3969/j.issn.1001-5256.2021.06.047. 期刊类型引用(1)
1. 李帅,王娟,夏仕雪,顾鹏. 子宫颈混合性腺神经内分泌癌胰腺转移1例. 中国医学影像学杂志. 2022(12): 1283-1284 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 709 KB)
PDF下载 ( 709 KB)


 下载:
下载:

 百度学术
百度学术
 下载:
下载: