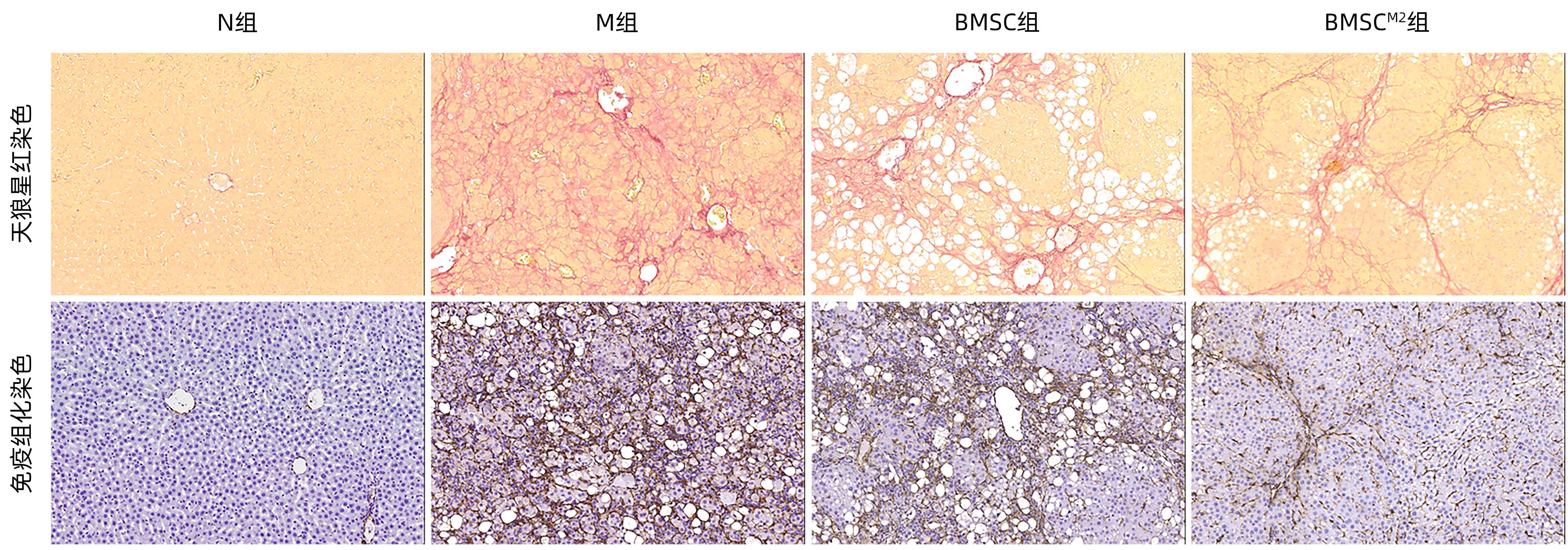
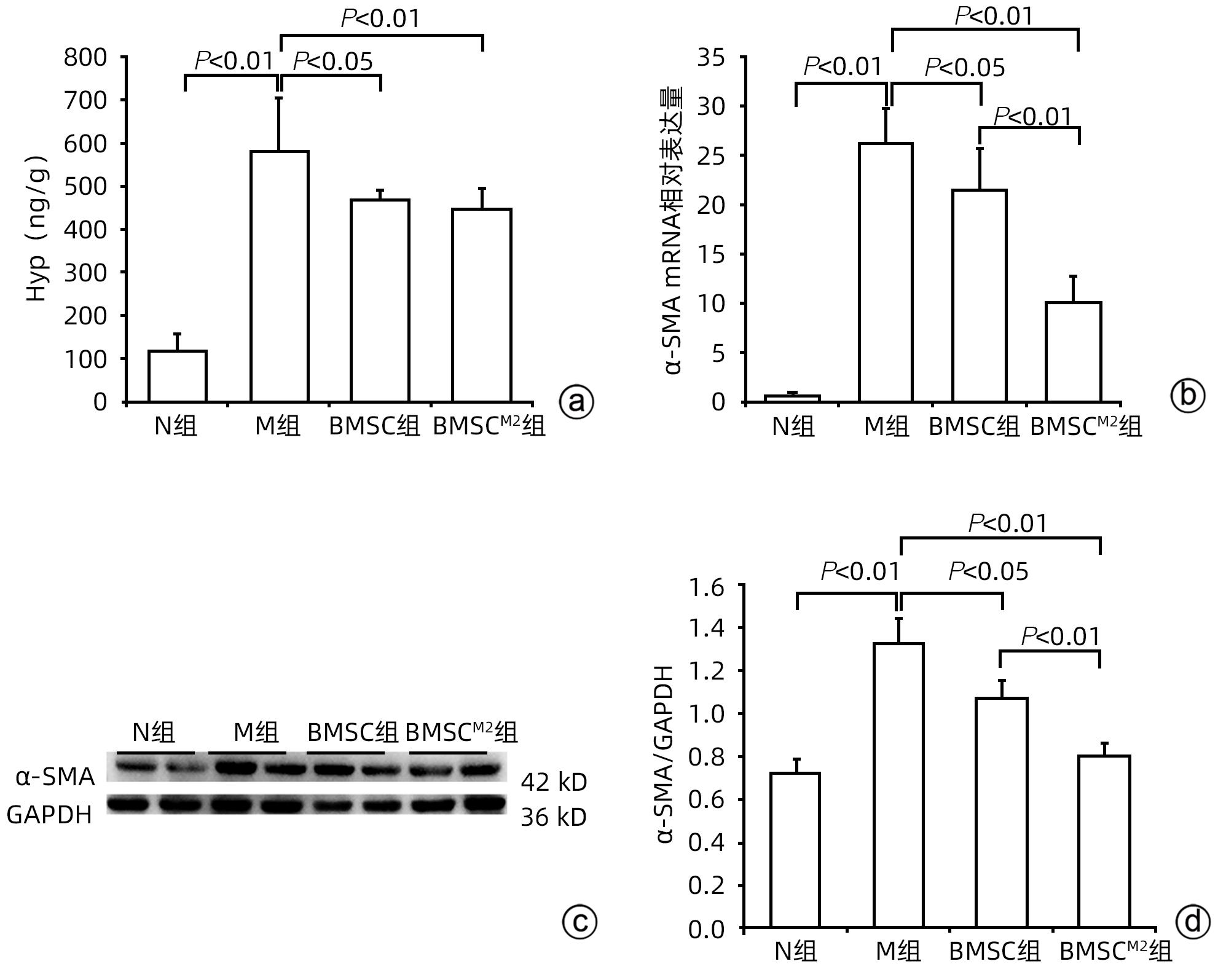
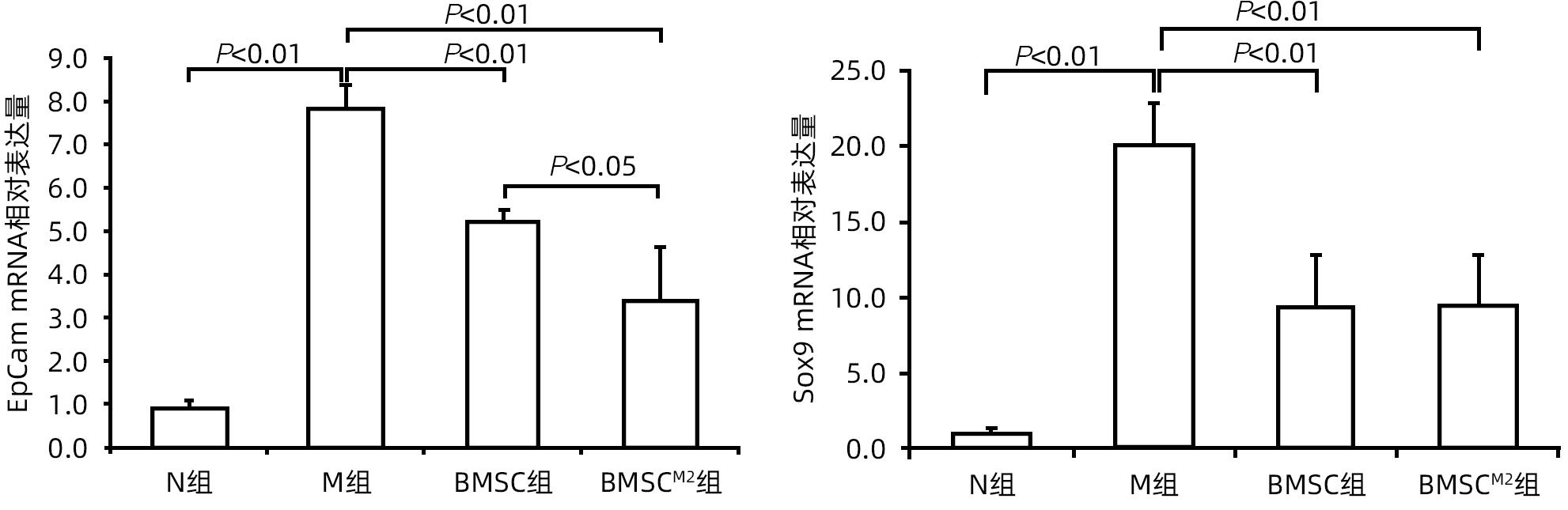
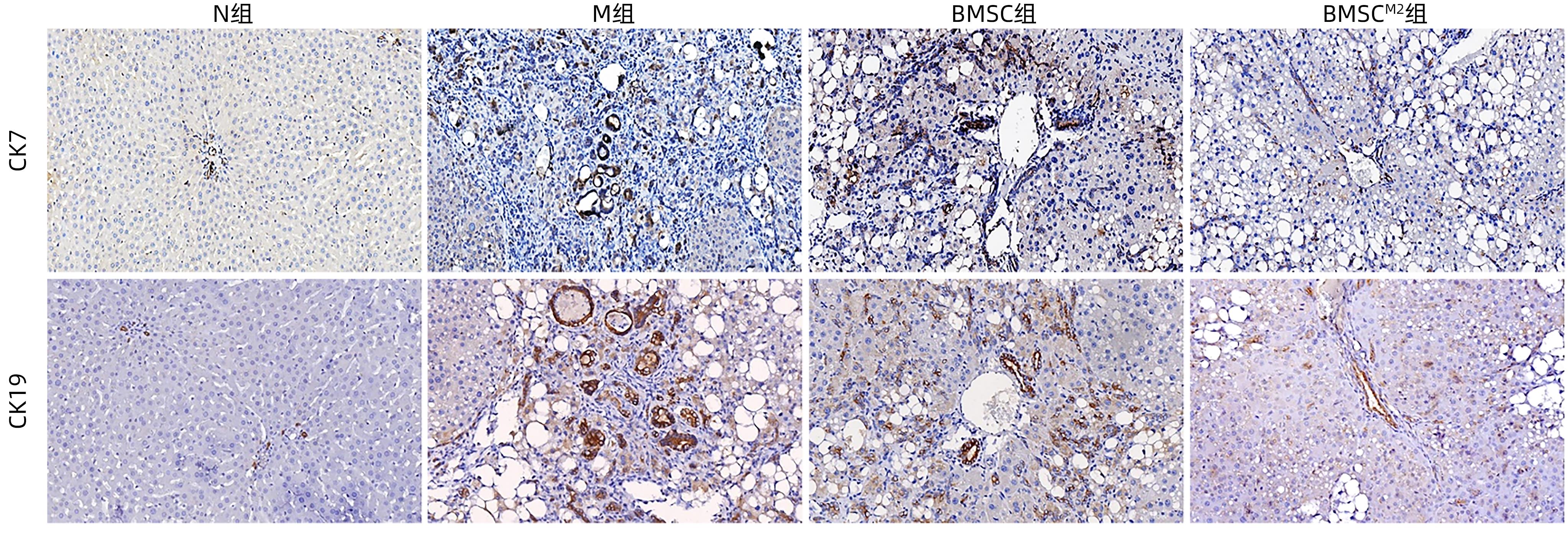
| [1] |
FREEMAN RB Jr, STEFFICK DE, GUIDINGER MK, et al. Liver and intestine transplantation in the United States, 1997-2006[J]. Am J Transplant, 2008, 8( 4 Pt 2): 958- 976. DOI: 10.1111/j.1600-6143.2008.02174.x.
|
| [2] |
ZHANG YT, LI YW, ZHANG LL, et al. Mesenchymal stem cells: Potential application for the treatment of hepatic cirrhosis[J]. Stem Cell Res Ther, 2018, 9( 1): 59. DOI: 10.1186/s13287-018-0814-4.
|
| [3] |
XIE RP, GU MQ, ZHANG FB, et al. Current status and prospect of surgical technique of liver transplantation[J]. Ogran Transplant, 2022, 13( 1): 105- 110. DOI: 10.3969/j.issn.1674-7445.2022.01.016.谢闰鹏, 谷明旗, 张凤博, 等. 肝移植手术技术的现状和展望[J]. 器官移植, 2022, 13( 1): 105- 110. DOI: 10.3969/j.issn.1674-7445.2022.01.016.
|
| [4] |
XIA Q, SHA M. Progress and prospect of living donor liver transplantation[J]. Chin J Dig Surg, 2022, 21( 1): 39- 42. DOI: 10.3760/cma.j.cn115610-20211205-00622.夏强, 沙朦. 活体肝移植的进展与展望[J]. 中华消化外科杂志, 2022, 21( 1): 39- 42. DOI: 10.3760/cma.j.cn115610-20211205-00622.
|
| [5] |
HUANG W, BHADURI A, VELMESHEV D, et al. Origins and proliferative states of human oligodendrocyte precursor cells[J]. Cell, 2020, 182( 3): 594- 608. DOI: 10.1016/j.cell.2020.06.027.
|
| [6] |
KHARAZIHA P, HELLSTRÖM PM, NOORINAYER B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial[J]. Eur J Gastroenterol Hepatol, 2009, 21( 10): 1199- 1205. DOI: 10.1097/MEG.0b013e32832a1f6c.
|
| [7] |
SUK KT, YOON JH, KIM MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial[J]. Hepatology, 2016, 64( 6): 2185- 2197. DOI: 10.1002/hep.28693.
|
| [8] |
ESMAEILZADEH A, OMMATI H, KOOSHYAR MM, et al. Autologous bone marrow stem cell transplantation in liver cirrhosis after correcting nutritional anomalies, A controlled clinical study[J]. Cell J, 2019, 21( 3): 268- 273. DOI: 10.22074/cellj.2019.6108.
|
| [9] |
JIA SS, LIU X, LI WY, et al. Peroxisome proliferator-activated receptor gamma negatively regulates the differentiation of bone marrow-derived mesenchymal stem cells toward myofibroblasts in liver fibrogenesis[J]. Cell Physiol Biochem, 2015, 37( 6): 2085- 2100. DOI: 10.1159/000438567.
|
| [10] |
JIAN X, WANG DY, XU YN, et al. Effect of polarized bone marrow-derived macrophage transplantation on the progression of CCl4-induced liver fibrosis in rats[J]. J Clin Hepatol, 2021, 37( 12): 2830- 2837. DOI: 10.3969/j.issn.1001-5256.2021.12.020.简迅, 王丹阳, 许燕楠, 等. 极化骨髓巨噬细胞移植对CCl4诱导的肝纤维化大鼠模型的影响[J]. 临床肝胆病杂志, 2021, 37( 12): 2830- 2837. DOI: 10.3969/j.issn.1001-5256.2021.12.020.
|
| [11] |
XU YN, XU W, ZHANG X, et al. BM-MSCs overexpressing the Numb enhance the therapeutic effect on cholestatic liver fibrosis by inhibiting the ductular reaction[J]. Stem Cell Res Ther, 2023, 14( 1): 45. DOI: 10.1186/s13287-023-03276-w.
|
| [12] |
JAMALL IS, FINELLI VN, QUE HEE SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues[J]. Anal Biochem, 1981, 112( 1): 70- 75. DOI: 10.1016/0003-2697(81)90261-x.
|
| [13] |
MU YP, OGAWA T, KAWADA N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine-choline-deficient diet[J]. Lab Invest, 2010, 90( 2): 245- 256. DOI: 10.1038/labinvest.2009.123.
|
| [14] |
PRADERE JP, KLUWE J, DE MINICIS S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice[J]. Hepatology, 2013, 58( 4): 1461- 1473. DOI: 10.1002/hep.26429.
|
| [15] |
KARLMARK KR, WEISKIRCHEN R, ZIMMERMANN HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis[J]. Hepatology, 2009, 50( 1): 261- 274. DOI: 10.1002/hep.22950.
|
| [16] |
VANNELLA KM, WYNN TA. Mechanisms of organ injury and repair by macrophages[J]. Annu Rev Physiol, 2017, 79: 593- 617. DOI: 10.1146/annurev-physiol-022516-034356.
|
| [17] |
ORECCHIONI M, GHOSHEH Y, PRAMOD AB, et al. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages[J]. Front Immunol, 2019, 10: 1084. DOI: 10.3389/fimmu.2019.01084.
|
| [18] |
ZHOU T, YUAN ZN, WENG JY, et al. Challenges and advances in clinical applications of mesenchymal stromal cells[J]. J Hematol Oncol, 2021, 14( 1): 24. DOI: 10.1186/s13045-021-01037-x.
|
| [20] |
NISHINA T, HOSHIKAWA KT, UENO Y. Current cell-based therapies in the chronic liver diseases[J]. Adv Exp Med Biol, 2018, 1103: 243- 253. DOI: 10.1007/978-4-431-56847-6_13.
|
| [21] |
SAITO Y, IKEMOTO T, TOKUDA K, et al. Effective three-dimensional culture of hepatocyte-like cells generated from human adipose-derived mesenchymal stem cells[J]. J Hepatobiliary Pancreat Sci, 2021, 28( 9): 705- 715. DOI: 10.1002/jhbp.1024.
|
| [22] |
LUO XY, MENG XJ, CAO DC, et al. Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes[J]. Stem Cell Res Ther, 2019, 10( 1): 16. DOI: 10.1186/s13287-018-1122-8.
|
| [23] |
CHAI NL, ZHANG XB, CHEN SW, et al. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats[J]. World J Gastroenterol, 2016, 22( 26): 6036- 6048. DOI: 10.3748/wjg.v22.i26.6036.
|
| [24] |
GHAFOURI-FARD S, NIAZI V, HUSSEN BM, et al. The emerging role of exosomes in the treatment of human disorders with a special focus on mesenchymal stem cells-derived exosomes[J]. Front Cell Dev Biol, 2021, 9: 653296. DOI: 10.3389/fcell.2021.653296.
|
| [25] |
FONDEVILA MF, FERNANDEZ U, HERAS V, et al. Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis[J]. J Hepatol, 2022, 77( 1): 15- 28. DOI: 10.1016/j.jhep.2022.02.003.
|
| [26] |
NOVO E, MARRA F, ZAMARA E, et al. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells[J]. Gut, 2006, 55( 1): 90- 97. DOI: 10.1136/gut.2005.069633.
|
| [27] |
WU XP, SHU LL, ZHANG ZX, et al. Adipocyte fatty acid binding protein promotes the onset and progression of liver fibrosis via mediating the crosstalk between liver sinusoidal endothelial cells and hepatic stellate cells[J]. Adv Sci, 2021, 8( 11): e2003721. DOI: 10.1002/advs.202003721.
|
| [28] |
PENG JY, LI F, WANG J, et al. Identification of a rare Gli1+ progenitor cell population contributing to liver regeneration during chronic injury[J]. Cell Discov, 2022, 8( 1): 118. DOI: 10.1038/s41421-022-00474-3.
|
| [29] |
TARLOW BD, FINEGOLD MJ, GROMPE M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury[J]. Hepatology, 2014, 60( 1): 278- 289. DOI: 10.1002/hep.27084.
|
| [30] |
MISHRA L, BANKER T, MURRAY J, et al. Liver stem cells and hepatocellular carcinoma[J]. Hepatology, 2009, 49( 1): 318- 329. DOI: 10.1002/hep.22704.
|
| [31] |
CHEN JM, ZHANG X, XU Y, et al. Hepatic progenitor cells contribute to the progression of 2-acetylaminofluorene/carbon tetrachloride-induced cirrhosis via the non-canonical Wnt pathway[J]. PLoS One, 2015, 10( 6): e0130310. DOI: 10.1371/journal.pone.0130310.
|
| [32] |
YAGI K, KOJIMA M, OYAGI S, et al. Application of mesenchymal stem cells to liver regenerative medicine[J]. Yakugaku Zasshi, 2008, 128( 1): 3- 9. DOI: 10.1248/yakushi.128.3.
|



 PDF下载 ( 4389 KB)
PDF下载 ( 4389 KB)

 下载:
下载: