胰腺导管腺癌行腹腔镜下胰十二指肠切除术后早期复发的列线图模型及其预测价值分析
DOI: 10.12449/JCH240123
Value of a nomogram model in early recurrence of pancreatic ductal adenocarcinoma after laparoscopic pancreaticoduodenectomy
-
摘要:
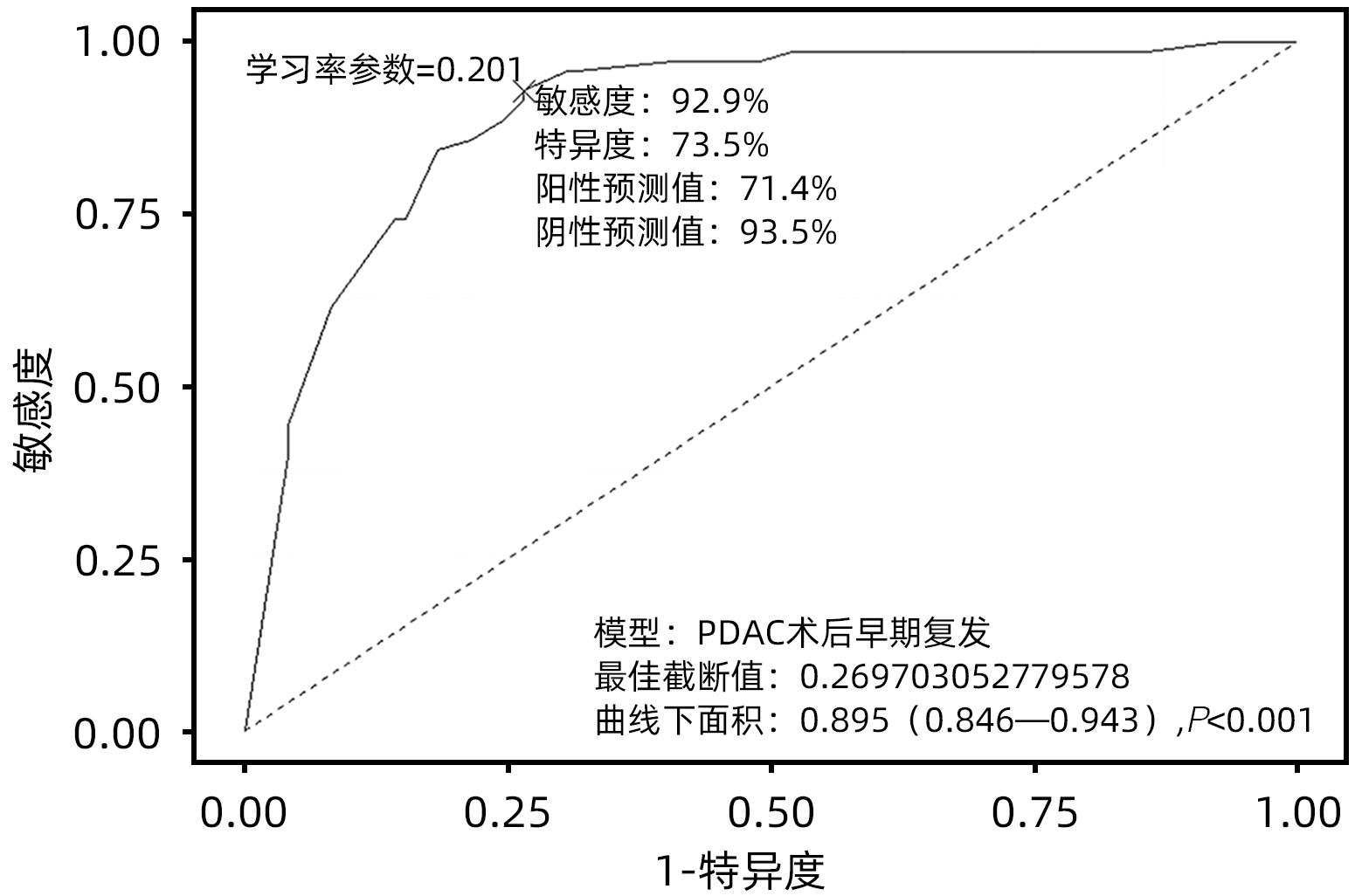
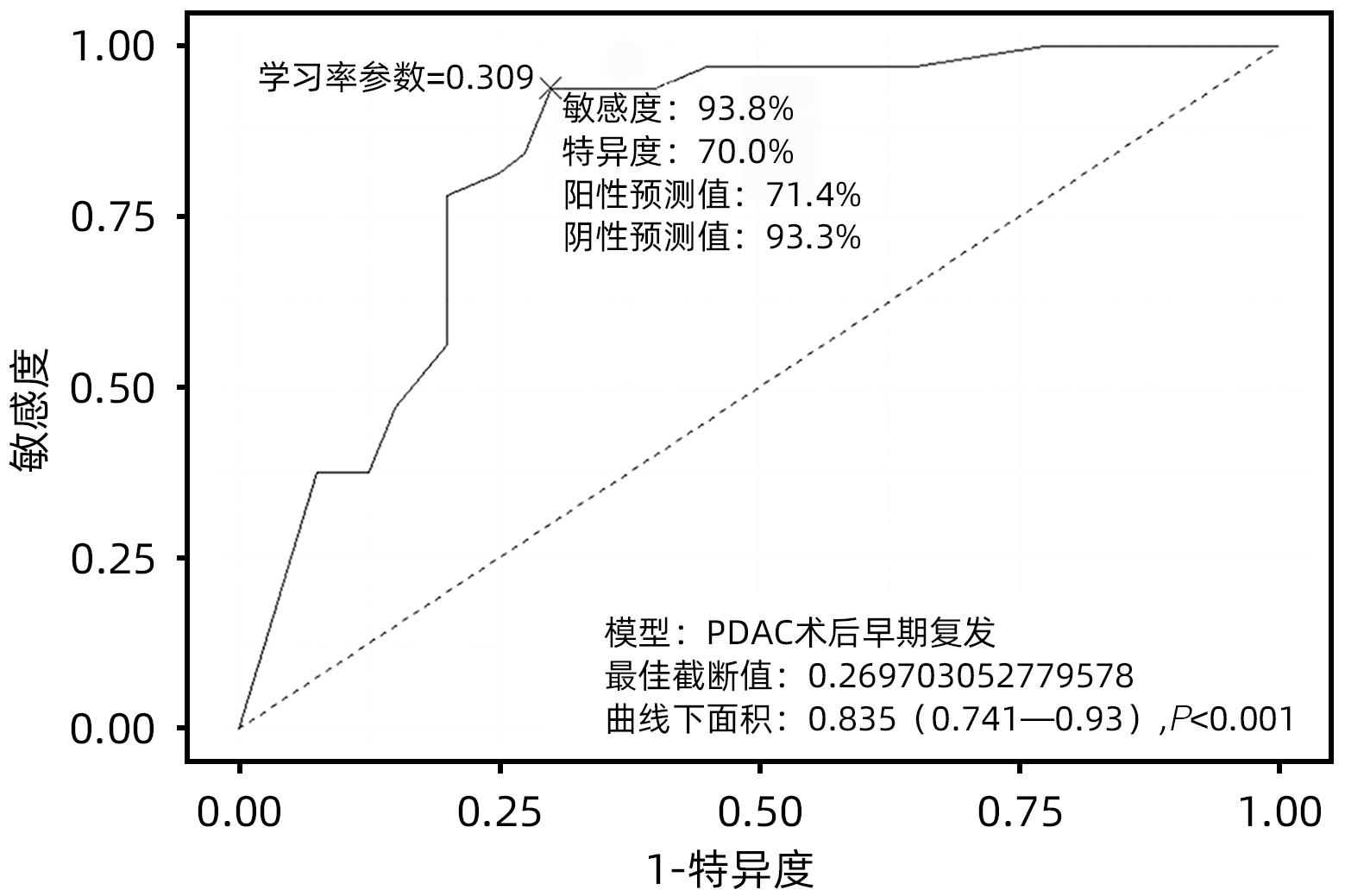
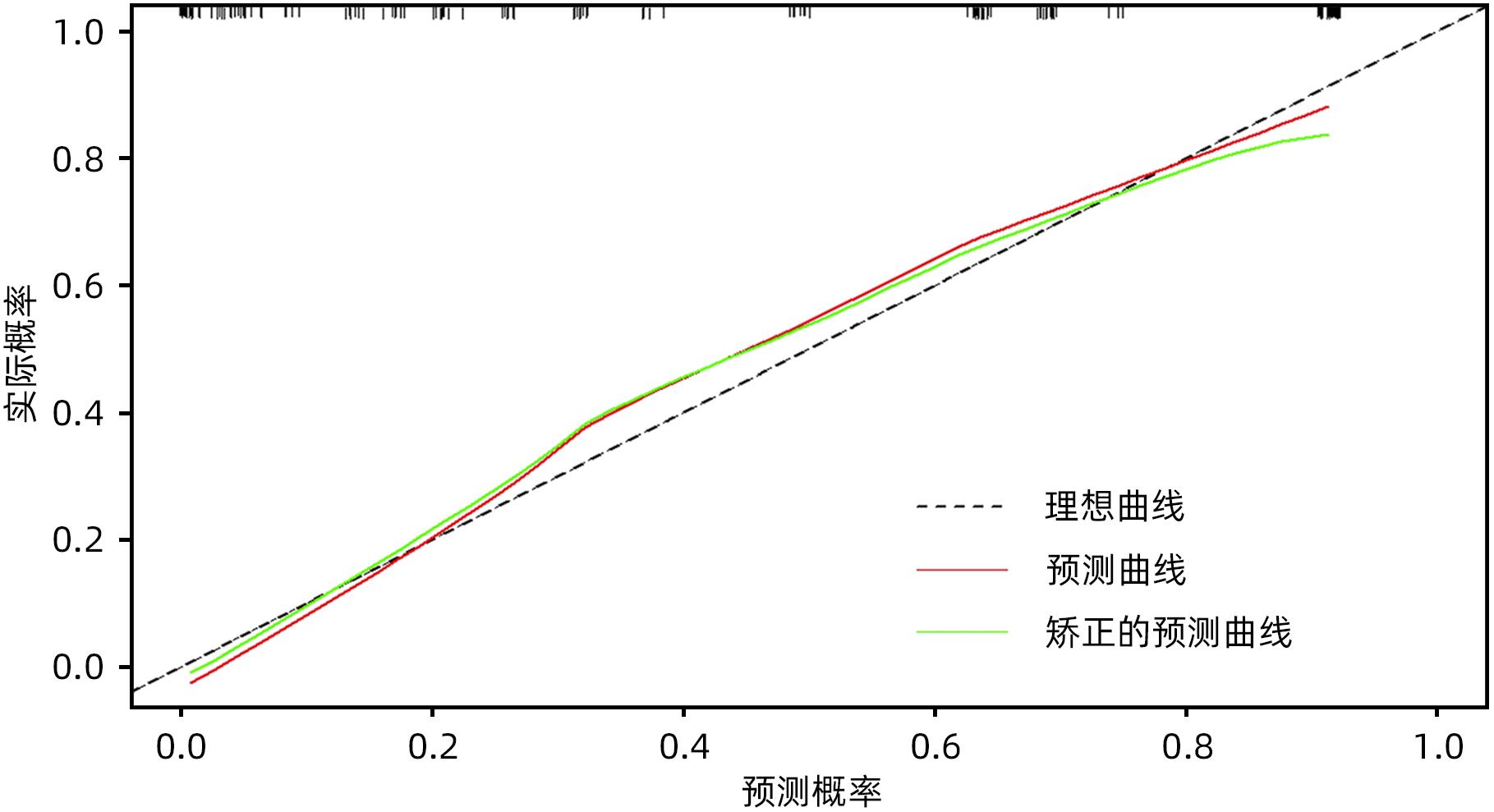
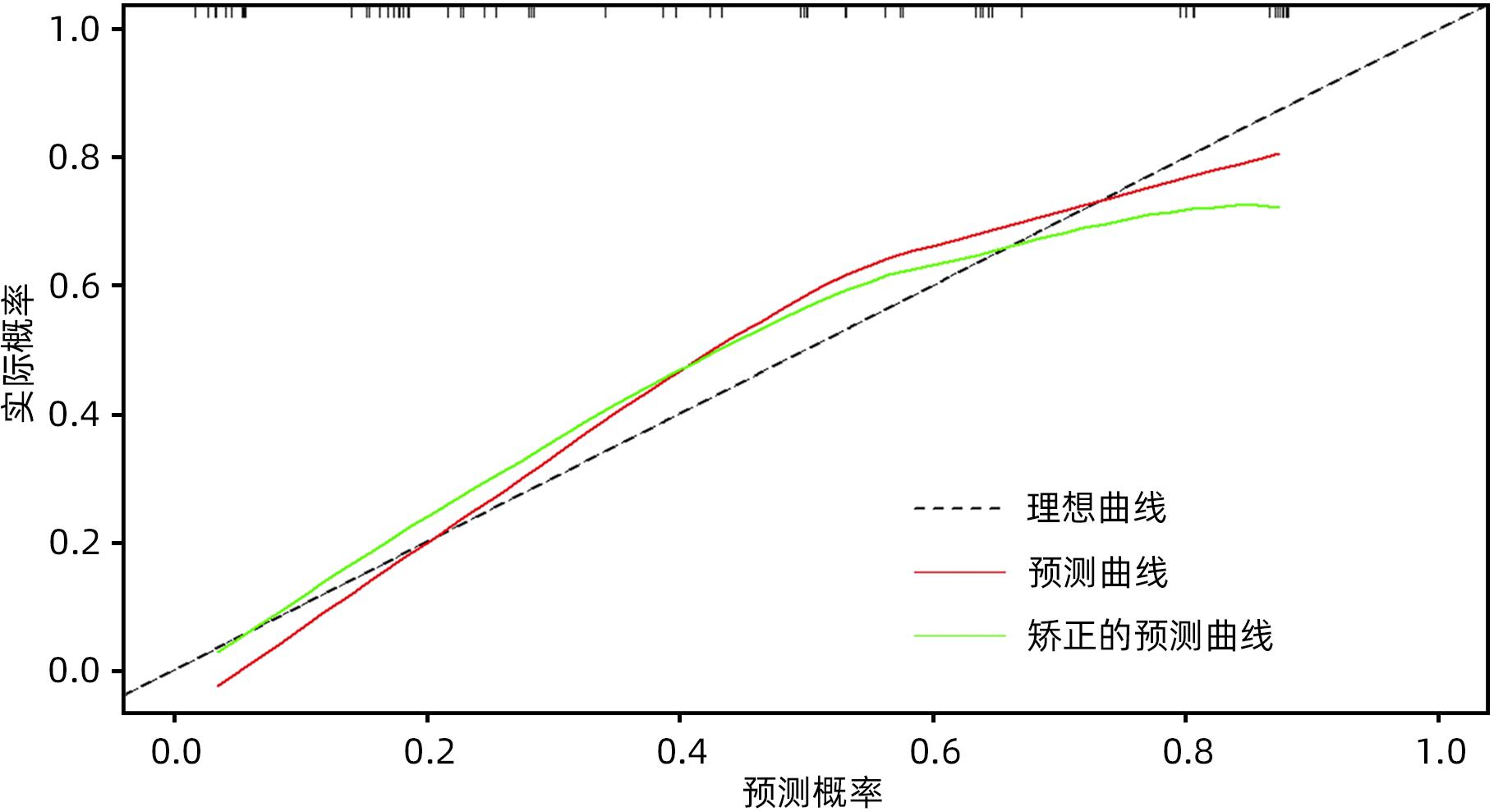
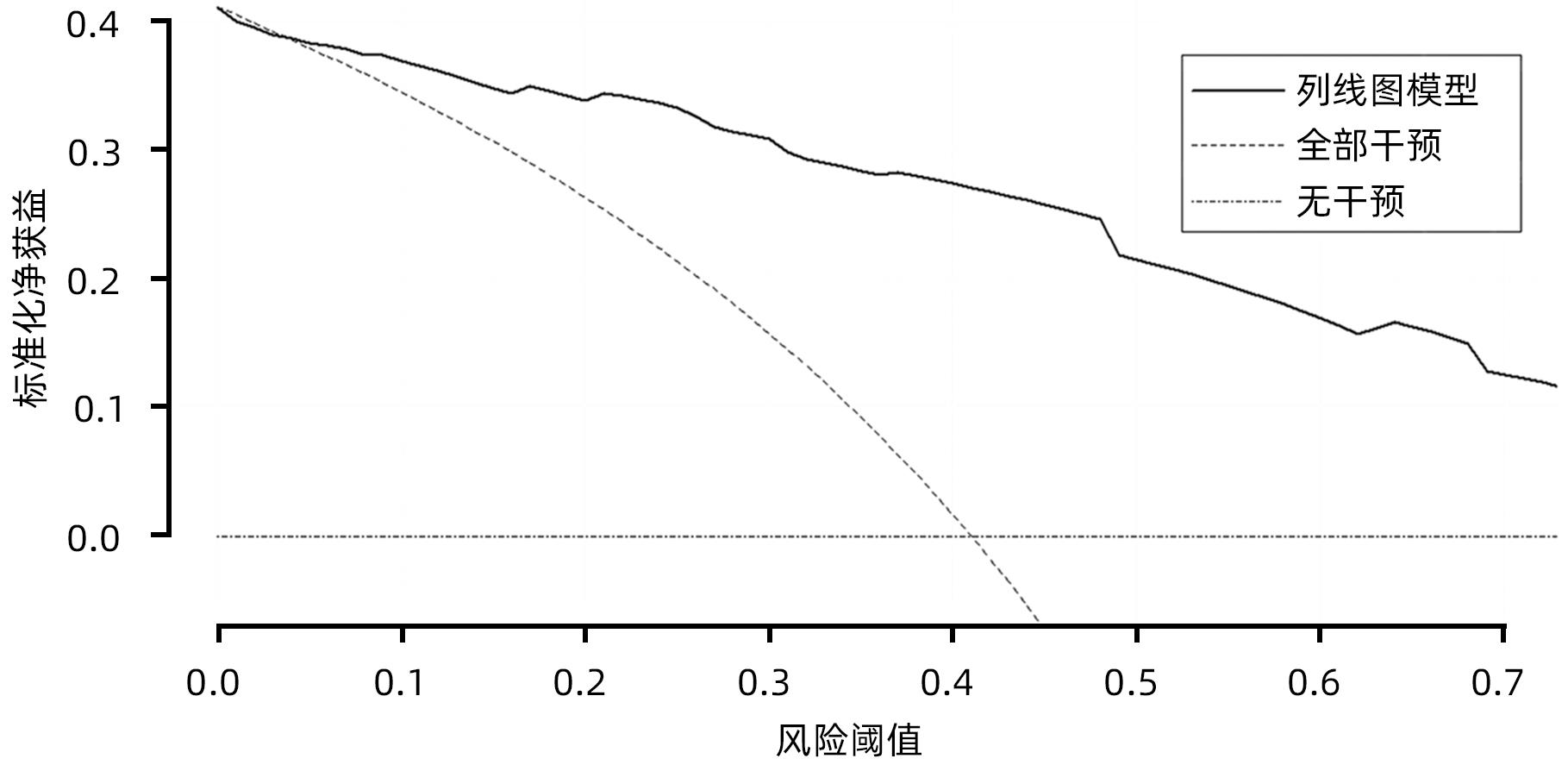
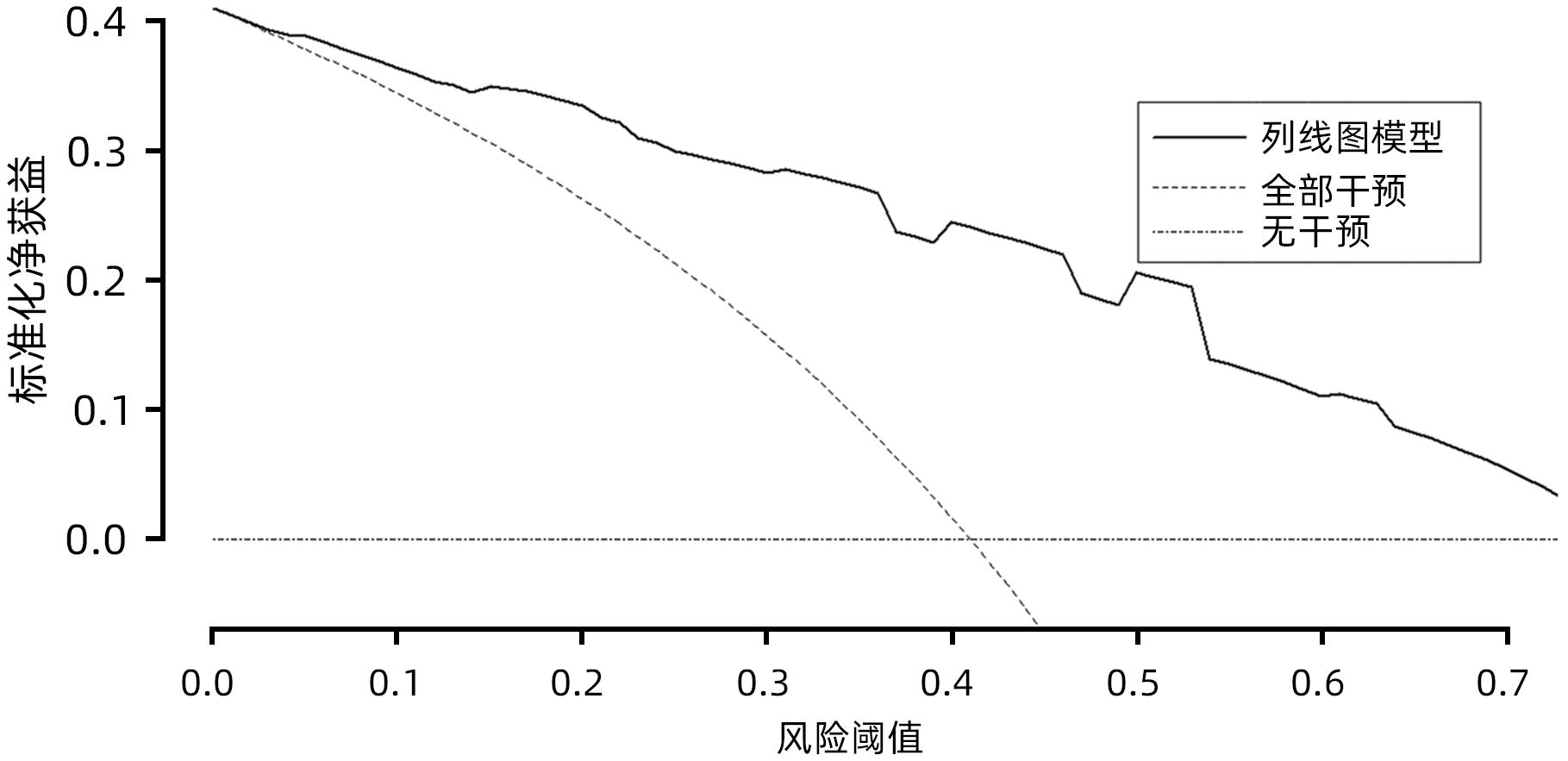
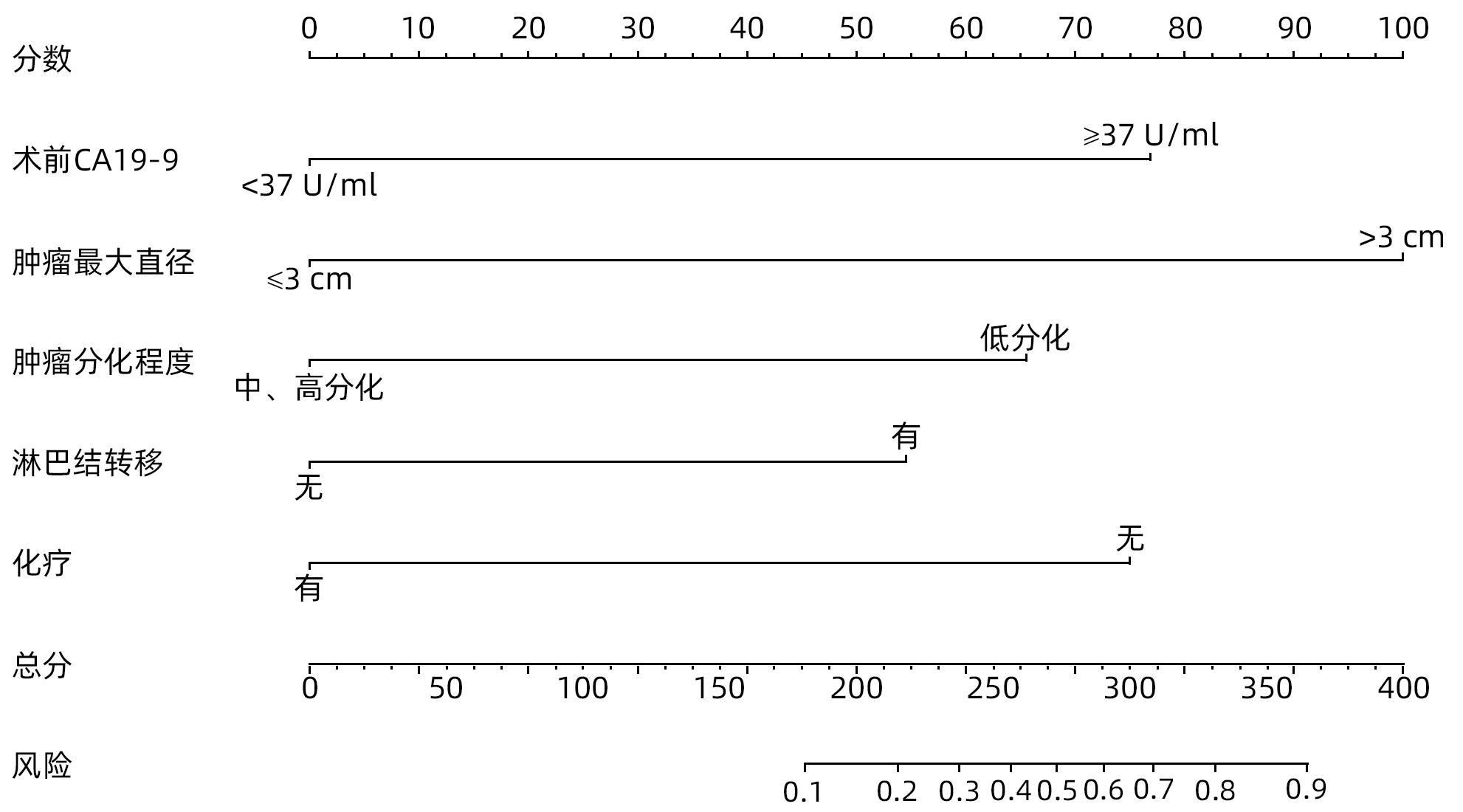
目的 探究胰腺导管腺癌(PDAC)患者行腹腔镜胰十二指肠切除术(LPD)后肿瘤早期复发的危险因素并建立预测模型。 方法 回顾性分析2016年4月—2022年7月于吉林大学第一医院行LPD的240例PDAC患者的临床资料,以术后肿瘤早期复发(复发时间≤12个月)为研究结局。按照7∶3比例,随机将患者分为训练组(n=168)与验证组(n=72)。训练组术后早期复发70例(41.67%),非早期复发98例(58.33%)。验证组术后早期复发32例(44.44%),非早期复发40例(55.56%)。计数资料组间比较采用χ2检验或Fisher精确概率法。Logistic回归分析影响术后早期复发的危险因素。采用受试者工作特征曲线下面积(AUC)评估模型的区分度,AUC>0.75为该模型有足够的区分度。用Bootstrap重采样法随机抽样1 000次验证,并用验证组再次验证。使用校准曲线和Hosmer-Lemeshow拟合优度检验评估校准度,决策曲线评估临床实用性。 结果 单因素、多因素分析结果显示:术前CA19-9水平≥37 U/mL、肿瘤最大直径>3 cm、肿瘤低分化、有淋巴结转移、术后未行辅助化疗是影响PDAC行LPD术后早期复发的独立危险因素[OR(95%CI)分别为6.265(1.938~20.249)、10.878(4.090~28.932)、3.679(1.435~9.433)、0.209(0.080~0.551)、0.167(0.058~0.480),P值均<0.05]。以此为基础构建列线图模型,AUC=0.895(95%CI:0.846~0.943,P<0.001),校准曲线Hosmer-Lemeshow检验表明模型具有良好的校准度(P=0.173)。决策曲线显示列线图具有良好的临床应用价值。 结论 术前CA19-9水平≥37 U/mL、肿瘤最大直径>3 cm、肿瘤低分化、有淋巴结转移、术后未行辅助化疗是影响PDAC LPD术后早期复发的独立危险因素,以此为依据构建列线图模型可有效预测术后早期复发。 Abstract:Objective To investigate the risk factors for early tumor recurrence after laparoscopic pancreaticoduodenectomy (LPD) in patients with pancreatic ductal adenocarcinoma (PDAC), and to establish a predictive model. Methods A retrospective analysis was performed for the clinical data of 240 PDAC patients who underwent LPD in The First Hospital of Jilin University from April 2016 to July 2022, with early postoperative tumor recurrence (time to recurrence ≤12 months) as the study outcome. The patients were randomly divided into training group with 168 patients and validation group with 72 patients at a ratio of 7∶3. In the training group, there were 70 patients (41.67%) with early postoperative recurrence and 98 (58.33%) without early recurrence, and in the validation group, there were 32 (44.44%) with early postoperative recurrence and 40 (55.56%) without early recurrence. The chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups; a logistic regression analysis was used to investigate the risk factors for early postoperative recurrence; the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to evaluate the discriminatory ability of the model, with AUC>0.75 indicating that the model had adequate discriminatory ability. The Bootstrap resampling method was used for validation after 1 000 times of random sampling, and the model was validated again in the validation group. The calibration curve and the Hosmer-Lemeshow goodness-of-fit test were used to evaluate the degree of calibration, and the decision curve analysis was used to evaluate clinical practicability. Results The univariate and multivariate analyses showed that preoperative CA19-9 level≥37 U/mL (odds ratio [OR]=6.265, 95% confidence interval [CI]: 1.938 — 20.249, P<0.05), maximum tumor diameter >3 cm (OR=10.878, 95%CI: 4.090 — 28.932, P<0.05), poor tumor differentiation (OR=3.679, 95%CI: 1.435 — 9.433, P<0.05), lymph node metastasis (OR=0.209, 95%CI: 0.080 — 0.551, P<0.05), and absence of adjuvant chemotherapy after surgery (OR=0.167, 95%CI: 0.058 — 0.480, P<0.05). A nomogram model was constructed based on these factors; the ROC curve analysis showed that the model had an AUC of 0.895 (95%CI: 0.846 — 0.943, P<0.001), and the calibration curve and the Hosmer-Lemeshow test showed that the model had a good degree of calibration (P=0.173). The decision curve analysis showed that the nomogram had a good clinical application value. Conclusion Preoperative CA19-9 level ≥37 U/mL, maximum tumor diameter >3 cm, poor tumor differentiation, lymph node metastasis, and absence of adjuvant chemotherapy after surgery are independent risk factors for the early recurrence of PDAC after LPD, and the nomogram model established based on these factors can effectively predict early postoperative recurrence. -
表 1 训练组和验证组基线数据特征比较
Table 1. Comparison of baseline data characteristics between training group and verification group
项目 训练组(n=168) 验证组(n=72) χ²值 P值 <60岁/≥60岁(例) 88/80 35/37 0.287 0.592 男/女(例) 92/76 42/30 0.261 0.610 BMI[例(%)] 0.008 0.928 <24.0 kg/m3 113(67.26) 48(66.67) ≥24.0 kg/m3 55(32.74) 24(33.33) 高血压[例(%)] 29(17.26) 12(16.67) 0.013 0.911 糖尿病[例(%)] 37(22.02) 12(16.67) 0.890 0.345 上腹部手术史[例(%)] 17(10.12) 10(13.89) 0.717 0.397 术前CA19-9[例(%)] 0.512 0.474 <37 U/mL 46(27.38) 23(31.94) ≥37 U/mL 122(72.62) 49(68.06) 术前CA125[例(%)] 0.540 0.463 <35 U/mL 146(86.90) 65(90.28) ≥35 U/mL 22(13.10) 7(9.72) 术前总胆红素[例(%)] 0.003 0.953 ≤170 μmol /L 109(64.88) 47(65.28) >170 μmol /L 59(35.12) 25(34.72) 术前胆道引流[例(%)] 73(43.45) 25(34.72) 1.590 0.207 ASA[例(%)] 0.224 0.636 1+2 144(85.71) 60(83.33) 3+4 24(14.29) 12(16.67) 术中失血量[例(%)] 0.025 0.874 ≤400 mL 122(72.62) 53(73.61) >400 mL 46(27.38) 19(26.39) 术中输血[例(%)] 40(23.80) 18(25.00) 0.039 0.843 胰腺质地[例(%)] 0.167 0.682 软 63(37.50) 25(34.72) 硬 105(62.50) 47(65.28) 胰管直径[例(%)] 0.185 0.667 ≤3 mm 49(29.17) 23(31.94) >3 mm 119(70.83) 49(68.06) 胰瘘[例(%)] 15(8.93) 7(9.72) 0.038 0.845 胆瘘[例(%)] 4(2.38) 5(6.94) 2.908 0.088 延迟性胃瘫 [例(%)] 7(4.17) 3(4.17) 0.222 0.637 腹腔感染[例(%)] 10(5.95) 4(5.56) 0.085 0.771 肺部感染[例(%)] 3(1.79) 2(2.78) 0.6381) 术后出血[例(%)] 3(1.79) 5(6.94) 4.163 0.055 肿瘤最大直径[例(%)] 0.067 0.796 ≤3cm 67(39.88) 30(41.67) >3cm 101(60.12) 42(58.33) 肿瘤分化程度[例(%)] 0.013 0.909 低分化 97(57.74) 41(56.94) 中分化或高分化 71(42.26) 31(43.06) 淋巴结转移[例(%)] 109(64.88) 49(68.06) 0.226 0.635 神经浸润[例(%)] 142(84.52) 61(84.72) 0.002 0.969 脉管浸润[例(%)] 108(64.29) 45(62.50) 0.070 0.792 术后辅助化疗[例(%)] 49(29.17) 24(33.33) 0.413 0.520 注:1)为Fisher精确概率法。 表 2 PDAC患者行LPD术后早期复发单因素分析
Table 2. Univariate analysis of early recurrence after radical LPD in PDAC patients
项目 训练组(n=168) 验证组(n=72) χ²值 P值 <60岁/≥60岁(例) 47/51 41/29 1.844 0.175 男/女(例) 58/40 34/36 1.856 0.173 BMI[例(%)] 2.874 0.090 <24.0 kg/m3 71(72.45) 42(60.00) ≥24.0 kg/m3 27(27.55) 28(40.00) 高血压[例(%)] 18(18.37) 11(15.71) 0.201 0.654 糖尿病[例(%)] 20(20.41) 17(24.29) 0.357 0.550 上腹部手术史[例(%)] 12(12.24) 5(7.14) 1.169 0.280 术前总胆红素[例(%)] 0.269 0.604 ≤170 μmol /L 62(63.27) 47(67.14) >170 μmol /L 36(36.73) 23(32.86) 术前胆道引流[例(%)] 46(46.94) 27(38.57) 1.163 0.281 ASA[例(%)] 0.200 0.655 1+2 83(84.69) 61(87.14) 3+4 15(15.31) 9(12.86) 术前CA19-9[例(%)] 24.718 <0.001 <37 U/mL 41(41.84) 5(7.14) ≥37 U/mL 57(58.16) 65(92.86) 术前CA125[例(%)] 0.149 0.699 <35 U/mL 86(87.76) 60(85.71) ≥35 U/mL 12(12.24) 10(14.29) 术中失血量[例(%)] 0.168 0.682 ≤400 mL 70(71.43) 52(74.29) >400 mL 28(28.57) 18(25.71) 术中是否输血[例(%)] 23(23.47) 17(24.29) 0.015 0.903 胰腺质地[例(%)] 0.059 0.808 软 36(36.73) 27(38.57) 硬 62(63.27) 43(61.43) 胰管直径[例(%)] 0.297 0.586 ≤3 mm 27(27.55) 22(31.43) >3 mm 71(72.45) 48(68.57) 胰瘘[例(%)] 7(7.14) 8(11.43) 0.922 0.337 胆瘘[例(%)] 5(2.04) 2(2.86) 1.0001) 延迟性胃瘫[例(%)] 2(2.04) 5(7.14) 0.1291) 术后腹腔感染[例(%)] 5(5.10) 5(7.14) 0.304 0.743 术后肺部感染[例(%)] 2(2.04) 1(1.43) 1.0001) 术后出血[例(%)] 2(2.04) 1(1.43) 1.0001) 肿瘤最大直径[例(%)] 32.789 <0.001 ≤3 cm 57(58.16) 10(14.29) >3 cm 41(41.84) 60(85.71) 肿瘤分化程度[例(%)] 15.892 <0.001 低分化 44(44.90) 53(75.71) 中分化或高分化[例(%)] 54(55.10) 17(24.29) 淋巴结转移[例(%)] 49(50.00) 60(85.71) 22.858 <0.001 神经浸润[例(%)] 83(84.69) 59(84.29) 0.005 0.943 脉管浸润[例(%)] 64(65.31) 44(62.86) 0.107 0.744 术后辅助化疗[例(%)] 36(36.73) 13(18.57) 6.520 0.011 注:1)为Fisher精确概率法。 表 3 PDAC患者行LPD术后早期复发的多因素Logistic回归分析
Table 3. Multivariate Logistic regression analysis of early recurrence after radical LPD in PDAC patients
相关因素 偏回归系数 标准误差 Wald值 P值 OR 95%CI 术前CA19-9 1.835 0.599 9.398 0.002 6.265 1.938~20.249 肿瘤最大直径 2.387 0.499 22.869 <0.001 10.878 4.090~28.932 淋巴结转移 1.303 0.480 7.356 0.007 3.679 1.435~9.433 肿瘤分化程度 -1.563 0.493 10.037 0.002 0.209 0.080~0.551 术后辅助化疗 -1.791 0.539 11.035 0.001 0.167 0.058~0.480 常量 -3.167 0.721 19.279 <0.001 0.042 -
[1] KLEIN AP. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 7): 493- 502. DOI: 10.1038/s41575-021-00457-x. [2] RAHIB L, SMITH BD, AIZENBERG R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States[J]. Cancer Res, 2014, 74( 11): 2913- 2921. DOI: 10.1158/0008-5472.CAN-14-0155. [3] HALBROOK CJ, LYSSIOTIS CA, PASCA DI MAGLIANO M, et al. Pancreatic cancer: Advances and challenges[J]. Cell, 2023, 186( 8): 1729- 1754. DOI: 10.1016/j.cell.2023.02.014. [4] GE WY, WANG HX. Immunotherapy for pancreatic ductal adenocarcinoma: Challenges and opportunities[J]. J Clin Hepatol, 2019, 35( 5): 958- 963. DOI: 10.3969/j.issn.1001-5256.2019.05.005.葛伟玉, 王红霞. 胰腺导管腺癌的免疫治疗: 挑战与机遇并存[J]. 临床肝胆病杂志, 2019, 35( 5): 958- 963. DOI: 10.3969/j.issn.1001-5256.2019.05.005. [5] KLEEFF J, KORC M, APTE M, et al. Pancreatic cancer[J]. Nat Rev Dis Primers, 2016, 2: 16022. DOI: 10.1038/nrdp.2016.22. [6] HE J, AHUJA N, MAKARY MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades[J]. HPB, 2014, 16( 1): 83- 90. DOI: 10.1111/hpb.12078. [7] ZHANG XP, XU S, GAO YX, et al. Early and late recurrence patterns of pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: A multicenter study[J]. Int J Surg, 2023, 109( 4): 785- 793. DOI: 10.1097/JS9.0000000000000296. [8] JONES RP, PSARELLI EE, JACKSON R, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: A secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial[J]. JAMA Surg, 2019, 154( 11): 1038- 1048. DOI: 10.1001/jamasurg.2019.3337. [9] GROOT VP, REZAEE N, WU WC, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma[J]. Ann Surg, 2018, 267( 5): 936- 945. DOI: 10.1097/SLA.0000000000002234. [10] GROOT VP, GEMENETZIS G, BLAIR AB, et al. Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma[J]. Ann Surg Oncol, 2018, 25( 8): 2475- 2483. DOI: 10.1245/s10434-018-6558-7. [11] TEMPERO MA, MALAFA MP, AL-HAWARY M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 4): 439- 457. DOI: 10.6004/jnccn.2021.0017. [12] WANG SP, LIU SY, ZHANG W, et al. The value of“posterior approach, uncinate process priority, artery first” in laparoscopic pancreatoduodenectomy[J]. Natl Med J China, 2020, 100( 42): 3328- 3331. DOI: 10.3760/cma.j.cn112137-20200316-00789王树鹏, 刘松阳, 张威, 等.“ 后入路、钩突先行、动脉优先”在腹腔镜胰十二指肠切除术中的价值分析[J]. 中华医学杂志, 2020, 100( 42): 3328- 3331. DOI: 10.3760/cma.j.cn112137-20200316-00789 [13] WENTE MN, VEIT JA, BASSI C, et al. Postpancreatectomy hemorrhage(PPH): An International Study Group of Pancreatic Surgery(ISGPS) definition[J]. Surgery, 2007, 142( 1): 20- 25. DOI: 10.1016/j.surg.2007.02.001. [14] BASSI C, MARCHEGIANI G, DERVENIS C, et al. The 2016 update of the International Study Group(ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After[J]. Surgery, 2017, 161( 3): 584- 591. DOI: 10.1016/j.surg.2016.11.014. [15] KOCH M, GARDEN OJ, PADBURY R, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery[J]. Surgery, 2011, 149( 5): 680- 688. DOI: 10.1016/j.surg.2010.12.002. [16] Study Group of Pancreatic Surgery in Chinese Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of Chinese Research Hospital Association, Editorial Board of Chinese Journal of Surgery. A consensus statement on the diagnosis, treatment, and prevention of common complications after pancreatic surgery(2017)[J]. Chin J Surg, 2017, 55( 5): 328- 334. DOI: 10.3760/cma.j.issn.0529-5815.2017.05.003.中华医学会外科学分会胰腺外科学组, 中国研究型医院学会胰腺病专业委员会, 中华外科杂志编辑部. 胰腺术后外科常见并发症诊治及预防的专家共识(2017)[J]. 中华外科杂志, 2017, 55( 5): 328- 334. DOI: 10.3760/cma.j.issn.0529-5815.2017.05.003 [17] LOU WH, LIU YB, LIANG TB, et al. Expert consensus on diagnosis, treatment and prevention of common complications after pancreatic surgery(2017)[J]. Med J Peking Union Med Coll Hosp, 2017, 8( S1): 139- 146.楼文晖, 刘颖斌, 梁廷波, 等. 胰腺术后外科常见并发症诊治及预防的专家共识(2017)[J]. 协和医学杂志, 2017, 8( S1): 139- 146. [18] Chinese Ministry of Health. Diagnostic criteria for Nosocomial infection(Trial)[J]. Natl Med J China, 2001( 5): 61- 67. DOI: 10.3760/j.issn.0376-2491.2001.05.027.中华人民共和国卫生部. 医院感染诊断标准(试行)[J]. 中华医学杂志, 2001, 81( 5): 61- 67. DOI: 10.3760/j.issn.0376-2491.2001.05.027. [19] BULDANLI MZ, UÇANER B, ÇIFTÇI MS, et al. Laparoscopic Nissen fundoplication: A five-year single-center clinical experience and results[J]. Eur Rev Med Pharmacol Sci, 2023, 27( 4): 1346- 1351. DOI: 10.26355/eurrev_202302_31368. [20] BURASAKARN P, THIENHIRAN A, FUENGFOO P, et al. Analysis of preoperative risk factors for early recurrence after curative pancreatoduodenectomy for resectable pancreatic adenocarcinoma[J]. Innov Surg Sci, 2022, 7( 1): 5- 11. DOI: 10.1515/iss-2021-0034. [21] DAAMEN LA, DORLAND G, BRADA LJH, et al. Preoperative predictors for early and very early disease recurrence in patients undergoing resection of pancreatic ductal adenocarcinoma[J]. HPB, 2022, 24( 4): 535- 546. DOI: 10.1016/j.hpb.2021.09.004. [22] MATSUMOTO I, MURAKAMI Y, SHINZEKI M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: A multi-center retrospective study[J]. Pancreatology, 2015, 15( 6): 674- 680. DOI: 10.1016/j.pan.2015.09.008. [23] GROOT VP, GEMENETZIS G, BLAIR AB, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma[J]. Ann Surg, 2019, 269( 6): 1154- 1162. DOI: 10.1097/SLA.0000000000002734. [24] IMAMURA M, NAGAYAMA M, KYUNO D, et al. Perioperative predictors of early recurrence for resectable and borderline-resectable pancreatic cancer[J]. Cancers, 2021, 13( 10): 2285. DOI: 10.3390/cancers13102285. [25] WANG YW, CUI CH, LI MT, et al. Construction and validation of a nomogram prediction model for early recurrence of patients undergoing radical pancreaticoduodenectomy for pancreatic ductal adenocarcinoma[J]. Chin J Hepatobiliary Surg, 2023, 29( 7): 538- 543. DOI: 10.3760/cma.j.cn113884-20221028-00404王砚伟, 崔成昊, 李明泰, 等. 胰腺导管腺癌患者根治性PD术后早期复发列线图预测模型的构建与验证[J]. 中华肝胆外科杂志, 2023, 29( 7): 538- 543. DOI: 10.3760/cma.j.cn113884-20221028-00404 [26] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2016[J]. CA Cancer J Clin, 2016, 66( 1): 7- 30. DOI: 10.3322/caac.21332. [27] MA YS, YANG YM. An excerpt of pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up(2018)[J]. J Clin Hepatol, 2019, 35( 1): 67- 71. DOI: 10.3969/j.issn.1001-5256.2019.01.012马永蔌, 杨尹默.《2018年法国临床实践指南: 胰腺癌的诊断、治疗与随访》摘译[J]. 临床肝胆病杂志, 2019, 35( 1): 67- 71. DOI: 10.3969/j.issn.1001-5256.2019.01.012 [28] CHEN RF, ZHONG CR, ZHOU QB. Current status and perspectives of minimally invasive surgical treatment of pancreatic head carcinoma[J]. J Clin Hepatol, 2019, 35( 5): 953- 957. DOI: 10.3969/j.issn.1001-5256.2019.05.004.陈汝福, 钟诚锐, 周泉波. 胰头癌微创手术治疗的现状及展望[J]. 临床肝胆病杂志, 2019, 35( 5): 953- 957. DOI: 10.3969/j.issn.1001-5256.2019.05.004. [29] CHOI SH, KIM HY, HWANG HK, et al. Oncologic impact of local recurrence in resected pancreatic cancer and topographic preference in local recurrence patterns according to tumor location[J]. Pancreas, 2020, 49( 10): 1290- 1296. DOI: 10.1097/MPA.0000000000001679. [30] ZHANG TP, LIU YZ, REN B. Current status and challenges of total neoadjuvant therapy for pancreatic cancer[J]. Chin J Dig Surg, 2022, 21( 4): 461- 464. DOI: 10.3760/cma.j.cn115610-20220320-00141.张太平, 刘悦泽, 任博. 胰腺癌全程新辅助治疗的现状及挑战[J]. 中华消化外科杂志, 2022, 21( 4): 461- 464. DOI: 10.3760/cma.j.cn115610-20220320-00141. [31] ZHU LX, MAO L, DU J, et al. Clinical efficacy of radical resection of pancreatic cancer after neoadjuvant conversion therapy[J]. Chin J Dig Surg, 2023, 22( 7): 916- 923. DOI: 10.3760/cma.j.cn115610-20230512-00204.朱琳熙, 毛谅, 杜娟, 等. 胰腺癌新辅助转化治疗后根治性切除术的临床疗效[J]. 中华消化外科杂志, 2023, 22( 7): 916- 923. DOI: 10.3760/cma.j.cn115610-20230512-00204. [32] LUO GP, JIN KZ, DENG SM, et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter[J]. Biochim Biophys Acta Rev Cancer, 2021, 1875( 2): 188409. DOI: 10.1016/j.bbcan.2020.188409. [33] MAHAJAN UM, OEHRLE B, SIRTL S, et al. Independent validation and assay standardization of improved metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis[J]. Gastroenterology, 2022, 163( 5): 1407- 1422. DOI: 10.1053/j.gastro.2022.07.047. [34] ZHANG XP, GAO YX, XU S, et al. A novel online calculator to predict early recurrence and long-term survival of patients with resectable pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: A multicenter study[J]. Int J Surg, 2022, 106: 106891. DOI: 10.1016/j.ijsu.2022.106891. [35] XU S, ZHANG XP, ZHAO GD, et al. Development and validation of an online calculator to predict early recurrence and long-term survival in patients with distal cholangiocarcinoma after pancreaticoduodenectomy[J]. J Hepatobiliary Pancreat Sci, 2022, 29( 11): 1214- 1225. DOI: 10.1002/jhbp.1058. [36] CAPELLO M, BANTIS LE, SCELO G, et al. Sequential validation of blood-based protein biomarker candidates for early-stage pancreatic cancer[J]. J Natl Cancer Inst, 2017, 109( 4): djw266. DOI: 10.1093/jnci/djw266. [37] AZIZIAN A, RÜHLMANN F, KRAUSE T, et al. CA19-9 for detecting recurrence of pancreatic cancer[J]. Sci Rep, 2020, 10( 1): 1332. DOI: 10.1038/s41598-020-57930-x. [38] YOKOYAMA S, HAMADA T, HIGASHI M, et al. Predicted prognosis of patients with pancreatic cancer by machine learning[J]. Clin Cancer Res, 2020, 26( 10): 2411- 2421. DOI: 10.1158/1078-0432.CCR-19-1247. [39] ZHAO TS, XIAO D, JIN FJ, et al. ESE3-positive PSCs drive pancreatic cancer fibrosis, chemoresistance and poor prognosis via tumour-stromal IL-1β/NF-κB/ESE3 signalling axis[J]. Br J Cancer, 2022, 127( 8): 1461- 1472. DOI: 10.1038/s41416-022-01927-y. [40] OVERBEEK KA, GOGGINS MG, DBOUK M, et al. Timeline of development of pancreatic cancer and implications for successful early detection in high-risk individuals[J]. Gastroenterology, 2022, 162( 3): 772- 785.e4. DOI: 10.1053/j.gastro.2021.10.014. [41] HEID I, STEIGER K, TRAJKOVIC-ARSIC M, et al. Co-clinical assessment of tumor cellularity in pancreatic cancer[J]. Clin Cancer Res, 2017, 23( 6): 1461- 1470. DOI: 10.1158/1078-0432.CCR-15-2432. [42] YOU MS, LEE SH, CHOI YH, et al. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment[J]. BMC Cancer, 2019, 19( 1): 952. DOI: 10.1186/s12885-019-6193-0. [43] JAMG TOL, GOUMA DJ, BASSI C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery(ISGPS)[J]. Surgery, 2014, 156( 3): 591- 600. DOI: 10.1016/j.surg.2014.06.016. [44] General Office of National Health Commission. Standard for diagnosis and treatment of pancreatic cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 5): 1006- 1015. DOI: 10.3969/j.issn.1001-5256.2022.05.007.国家卫生健康委办公厅. 胰腺癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 5): 1006- 1015. DOI: 10.3969/j.issn.1001-5256.2022.05.007. [45] Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. Guidelines for the diagnosis and treatment of pancreatic cancer in China(2021)[J]. Chin J Pract Surg, 2021, 41( 7): 725- 738. DOI: 10.19538/j.cjps.issn1005-2208.2021.07.02.中华医学会外科学分会胰腺外科学组. 中国胰腺癌诊治指南(2021)[J]. 中国实用外科杂志, 2021, 41( 7): 725- 738. DOI: 10.19538/j.cjps.issn1005-2208.2021.07.02. -



 PDF下载 ( 1060 KB)
PDF下载 ( 1060 KB)

 下载:
下载: