索磷布韦/维帕他韦单用或联合利巴韦林治疗3B型HCV/HIV感染者的效果及安全性
DOI: 10.12449/JCH240209
Efficacy and safety of sofosbuvir/velpatasvir alone or in combination with ribavirin in treatment of patients with genotype 3B HCV/HIV infection
-
摘要:
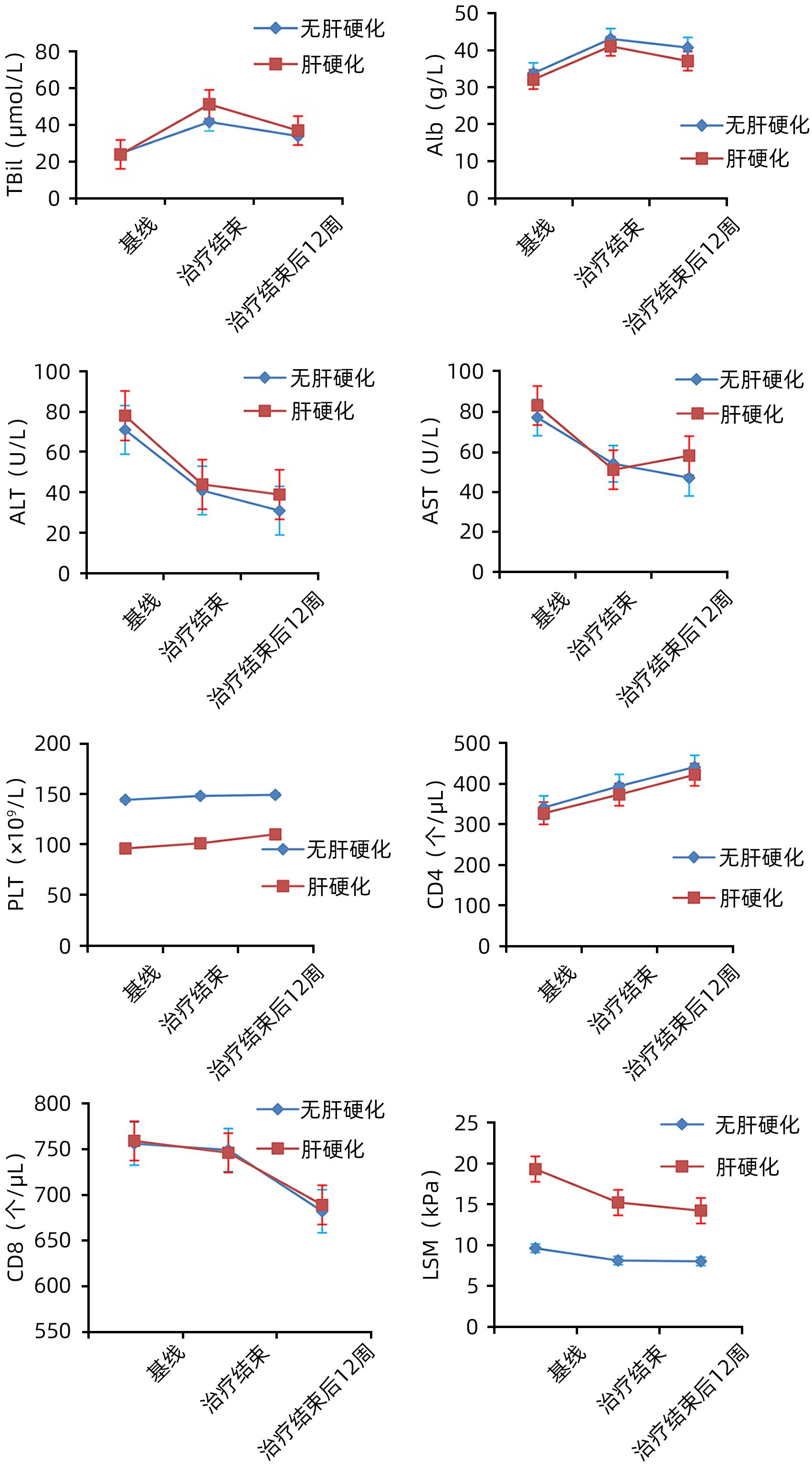
目的 观察索磷布韦/维帕他韦单用或联合利巴韦林方案对我国基因3B型HCV/HIV感染者的治疗效果和安全性。 方法 选取2017年1月—2020年12月于昆明市第三人民医院就诊的3B型HCV/HIV合并感染者299例,使用索磷布韦/维帕他韦单用或联合利巴韦林治疗12周,停药后随访12周。评估治疗结束后12周的持续病毒学应答率(SVR12)和不良反应。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。使用Agresti-Coull方法构建SVR12的95%CI。采用单因素和多因素非条件Logistic回归分析SVR的影响因素。 结果 299例3B型HCV/HIV感染者患者的平均年龄为(43.92±6.84)岁,男性占77.3%(231/299),肝硬化患者占36.5%(109/299),曾接受过抗病毒治疗者占13.4%(40/299),索磷布韦/维帕他韦联合利巴韦林治疗患者占27.8%(83/299)。患者总体SVR12为87.0%(260/299),其中索磷布韦/维帕他韦单用与联用利巴韦林SVR12比较,差异无统计学意义(87.5% vs 85.5%,χ2=0.203,P=0.653);无肝硬化和肝硬化患者SVR12比较,差异有统计学意义(90.0% vs 81.7%,χ2=4.256,P=0.039);抗病毒初治患者的SVR12明显高于经治患者(93.4% vs 45.0%,χ2=71.670,P<0.001)。单因素和多因素Logistic回归分析结果显示,PLT(OR=0.957,95%CI:0.931~0.984,P=0.002)、肝硬度值(OR=1.446,95%CI:1.147~1.822,P=0.002)和经治(OR=13.807,95%CI:2.970~64.174,P=0.001)是3B型HCV/HIV感染者SVR的独立影响因素。出现严重不良反应事件41例,均在抗病毒治疗后2周内出现,28例在没有停药并积极处理后缓解;13例积极处理后仍未缓解,停用抗病毒药物2~5 d后缓解,缓解后再次使用未出现类似反应。 结论 索磷布韦/维帕他韦单用或联合利巴韦林对3B型HCV/HIV感染者有较好的治疗效果和安全性。 Abstract:Objective To investigate the efficacy and safety of sofosbuvir/velpatasvir alone or in combination with ribavirin in Chinese patients with genotype 3B HCV/HIV infection. Methods A total of 299 patients with genotype 3B HCV/HIV infection who attended The Third People’s Hospital of Kunming from January 2017 to December 2020 were enrolled and treated with sofosbuvir/velpatasvir alone or in combination with ribavirin for 12 weeks, and they were followed up for 12 weeks after drug withdrawal. The patients were evaluated in terms of sustained virologic response at 12 weeks after treatment (SVR12) and adverse reactions. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups; the Agresti-Coull method was used to evaluate the 95% confidence interval (CI) of SVR12; univariate and multivariate non-conditional logistic regression analyses were used to investigate the influencing factors for SVR. Results The 299 patients with genotype 3B HCV/HIV infection had a mean age of 43.92±6.84 years, among whom the male patients accounted for 77.3% (231/299), the patients with liver cirrhosis accounted for 36.5% (109/299), the patients with a history of antiviral therapy accounted for 13.4% (40/299), and the patients receiving sofosbuvir/velpatasvir combined with ribavirin accounted for 27.8% (83/299). The overall SVR was 87.0% (260/299) for all patients, and there was no significant difference in SVR12 between the patients receiving sofosbuvir/velpatasvir alone and those receiving sofosbuvir/velpatasvir combined with ribavirin (87.5% vs 85.5%, χ2=0.203, P=0.653). There was a significant difference in SVR12 between the patients without liver cirrhosis and those with liver cirrhosis (90.0% vs 81.7%, χ2=4.256, P=0.039), and the patients receiving antiviral therapy for the first time had a significantly higher SVR12 than the treatment-experienced patients (93.4% vs 45.0%, χ2=71.670, P<0.001). The univariate and multivariate logistic regression analyses showed that platelet count (odds ratio [OR]=0.957, 95%CI: 0.931 — 0.984, P=0.002), liver stiffness measurement (OR=1.446, 95%CI: 1.147 — 1.822, P=0.002), and experience in treatment (OR=13.807, 95%CI: 2.970 — 64.174, P=0.001) were independent influencing factors for SVR in patients with genotype 3B HCV/HIV infection. There were 41 cases of serious adverse events, all of which occurred within 2 weeks after antiviral therapy, and 28 cases were resolved without drug withdrawal or active treatment, while 13 cases were not resolved after active treatment and were resolved after the antiviral drugs were stopped for 2 — 5 days, with no similar reactions observed when the drugs were used again after remission. Conclusion Sofosbuvir/velpatasvir alone or in combination with ribavirin has relatively good efficacy and safety in patients with genotype 3B HCV/HIV infection. -
Key words:
- Hepacivirus /
- HIV /
- Sofosbuvir /
- Velpatasvir /
- Ribavirin
-
表 1 索磷布韦/维帕他韦±利巴韦林治疗的HCV/HIV患者基线特征
Table 1. Baseline characteristics of patients who received sofosbuvir/velpatasvir±ribavirin with HCV/HIV
项目 患者合计 索磷布韦/维帕他韦组 (n=216) 索磷布韦/维帕他韦+利巴韦林组(n=83) 统计值 P值 男/女(例) 231/68 163/53 68/15 χ2=1.426 0.232 年龄(岁) 43.92±6.84 43.86±6.85 44.08±6.87 t=-0.258 0.797 BMI(kg/m2) 20.66±2.94 20.77±3.03 20.38±2.68 t=1.030 0.304 WBC(×109/L) 4.74(3.44~6.03) 4.95(3.56~6.38) 4.24(3.30~5.49) Z=-2.634 0.008 PLT(×109/L) 125(86~158) 131(94~168) 106(63~129) Z=-5.106 <0.001 TBil(μmol/L) 15.0(9.6~27.9) 14.7(8.9~26.1) 15.7(10.9~31.1) Z=-1.779 0.075 ALT(U/L) 55(30~85) 55(30~85) 59(29~89) Z=-0.409 0.682 AST(U/L) 63(40~84) 62(40~83) 66(41~86) Z=-0.695 0.487 Alb(g/L) 33.5±7.7 33.7±7.7 33.0±7.8 t=0.734 0.464 PAB(mg/L) 153(99~190) 157(102~194) 148(97~190) Z=-0.916 0.360 血糖(mmol/L) 5.96±1.74 5.90±1.38 6.01±2.46 t=-0.898 0.370 甘油三酯(mmol/L) 1.30(0.94~2.01) 1.32(0.94~2.01) 1.30(0.94~1.96) Z=-0.088 0.930 总胆固醇(mmol/L) 3.16±0.98 3.20±1.02 3.04±0.84 t=1.263 0.208 ALP(U/L) 98(75~136) 97(76~133) 102(75~137) Z=-0.683 0.494 GGT(U/L) 106(54~195) 108(53~199) 100(55~177) Z=-0.084 0.933 尿素氮(mmol/L) 4.60(3.40~6.50) 4.60(3.40~6.38) 4.70(3.50~6.90) Z=-0.970 0.332 肌酐(μmol/L) 67(56~81) 66(56~83) 68(54~79) Z=-0.371 0.710 β2微球蛋白(μg/mL) 3.93±1.71 3.86±1.72 4.13±1.66 t=-1.259 0.209 INR 1.22±0.24 1.20±0.21 1.28±0.31 t=-2.493 0.013 AFP(ng/mL) 5.21(2.90~17.06) 5.29(2.79~18.12) 5.09(3.05~15.78) Z=-0.291 0.771 异常凝血酶原(mAU/mL) 41(29~392) 40(29~220) 47(29~549) Z=-1.218 0.223 CD4(个/μL) 274(171~483) 296(171~508) 248(169~410) Z=-1.854 0.064 CD8(个/μL) 680(557~850) 689(564~858) 643(530~818) Z=-0.961 0.336 HCV RNA(log10 IU/mL) 5.95±1.11 5.93±1.13 5.99±1.04 t=-0.434 0.664 LSM(kPa) 11.4(9.2~16.6) 10.3(8.8~11.7) 18.5(14.8~21.1) Z=-11.234 <0.001 肝硬化(否/是,例) 190/109 190/26 0/83 χ2=200.273 <0.001 Child-Pugh(A/B/C,例) 65/19/25 12/6/8 53/13/17 χ2=202.608 <0.001 治疗史(初治/经治,例) 259/40 194/22 65/18 χ2=6.845 0.009 肝癌(否/是,例) 280/19 200/16 80/3 χ2=1.450 0.229 糖尿病(否/是,例) 281/18 200/16 81/2 χ2=2.647 0.104 高血压(否/是,例) 258/41 189/27 69/14 χ2=0.967 0.326 肾病(否/是,例) 282/17 201/15 81/2 χ2=2.299 0.129 心脏病(否/是,例) 258/41 190/26 68/15 χ2=1.846 0.174 脑病(否/是,例) 267/32 193/23 74/9 χ2=0.002 0.961 饮酒(否/是,例) 283/16 207/9 76/7 χ2=2.156 0.142 脂肪肝(否/是,例) 288/11 206/10 82/1 χ2=1.985 0.159 注:PAB,前白蛋白。 表 2 不同特征的HCV/HIV感染者的SVR12
Table 2. Sustained virologic respose at week 12 after end-of-treatment rates with HCV/HIV
亚组 SVR12(%) 95%CI(%) χ2值 P值 治疗方案 0.203 0.653 索磷布韦/韦帕他韦 87.5(189/216) 82.9~91.2 索磷布韦/韦帕他韦+利巴韦林 85.5(71/83) 78.3~92.8 性别 0.127 0.722 男 86.6(200/231) 82.3~90.9 女 88.2(60/68) 79.4~95.6 年龄 0.015 0.903 <50岁 86.9(218/251) 82.5~90.8 ≥50岁 87.5(42/48) 77.1~95.8 肝硬化 4.256 0.039 无 90.0(171/190) 85.8~94.2 有 81.7(89/109) 74.3~88.1 肝癌 0.311 0.564 否 86.8(243/280) 82.9~90.4 是 89.5(17/19) 73.7~100.0 治疗史 71.670 <0.001 初治 93.4(242/259) 90.3~96.1 经治 45.0(18/40) 30.0~60.0 肾功能不全 1.747 0.186 否 87.6(247/282) 83.7~91.1 是 76.5(13/17) 52.9~94.1 糖尿病 0.063 0.802 否 86.8(244/281) 82.6~90.7 是 88.9(16/18) 72.2~100.0 病毒载量 0.059 0.809 <5.9 log10 IU/mL 86.4(108/125) 80.3~92.5 ≥5.9 log10 IU/mL 87.4(152/174) 82.4~92.3 表 3 单因素和多因素Logistic分析
Table 3. Single factor and multi factor Logistic analysis
项目 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 男性 1.162(0.507~2.663) 0.722 年龄(岁) 1.008(0.960~1.058) 0.760 BMI(kg/m2) 1.002(0.894~1.124) 0.969 WBC(×109/L) 0.728(0.592~0.896) 0.031 0.868(0.583~1.293) 0.485 PLT(×109/L) 0.943(0.927~0.959) <0.001 0.957(0.931~0.984) 0.002 TBil(μmol/L) 1.007(0.998~1.016) 0.109 ALT(U/L) 0.993(0.985~1.001) 0.098 AST(U/L) 0.997(0.991~1.004) 0.430 Alb(g/L) 0.934(0.894~0.972) 0.003 1.084(0.966~1.216) 0.172 PAB(mg/L) 0.993(0.987~0.998) 0.013 0.993(0.979~1.007) 0.301 血糖(mmol/L) 0.861(0.654~1.133) 0.285 甘油三酯(mmol/L) 0.899(0.734~1.099) 0.299 总胆固醇(mmol/L) 0.884(0.619~1.263) 0.497 ALP(U/L) 0.998(0.993~1.003) 0.436 GGT(U/L) 0.996(0.992~1.000) 0.027 0.997(0.993~1.001) 0.141 尿素氮(mmol/L) 0.982(0.897~1.074) 0.691 肌酐(μmol/L) 0.995(0.984~1.007) 0.410 β2微球蛋白(μg/mL) 1.060(0.872~1.287) 0.560 INR 4.249(1.285~14.054) 0.018 0.663(0.074~5.936) 0.714 AFP(ng/mL) 1.000(0.999~1.000) 0.660 异常凝血酶原(mAU/mL) 1.016(0.995~1.038) 0.143 CD4(个/μL) 0.999(0.997~1.001) 0.220 CD8(个/μL) 0.999(0.998~1.001) 0.161 HCV RNA(log10 IU/mL) 0.792(0.596~1.051) 0.106 LSM(kPa) 1.101(1.048~1.156) <0.001 1.446(1.147~1.822) 0.002 治疗史(初治/经治) 17.399(7.868~38.476) <0.001 13.807(2.970~64.174) 0.001 肝癌(否/是) 1.001(0.889~1.145) 0.998 糖尿病(否/是) 0.824(0.182~3.732) 0.802 肾病(否/是) 2.171(0.670~7.033) 0.196 利巴韦林(未联合/联合) 1.183(0.569~2.462) 0.653 表 4 治疗失败的39例患者的特征
Table 4. Characteristic analysis of 39 cases of anti-virus failure
项目 数值 男/女(例) 31/8 年龄(岁) 44.23±6.27 WBC(×109/L) 4.05±1.62 PLT(×109/L) 58(40~64) Alb(g/L) 29.96±7.55 GGT(U/L) 74(40~158) INR 1.31±0.37 CD4(个/μL) 251(115~460) HCV RNA(log10 IU/mL) 5.68±1.14 LSM(kPa) 20.08±5.41 经治/初治(例) 22/17 肝硬化(是/否,例) 20/19 联合利巴韦林(是/否,例) 12/27 表 5 治疗的不良反应
Table 5. Treatment tolerability
项目 例(%) 严重不良事件 41(13.7) 不良事件 贫血 61(20.4) 疲乏 55(18.4) 恶心 28(9.4) 头痛 23(7.7) 食欲减退 24(8.0) 瘙痒 15(5.0) 失眠 9(3.0) 关节痛 9(3.0) 皮疹 17(5.7) 肌痛 10(3.3) 脱发 52(17.4) 腹泻 20(6.7) 其他 24(8.0) -
[1] World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection[M]. Geneva: World Health Organization, 2018. [2] World Health Organization. Global hepatitis report[R]. Geneva: WHO, 2017. [3] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35( 12): 2670- 2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2670- 2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. [4] XU AQ, ZHANG L. The review and significance of national seroepidemiological surveys on viral hepatitis in China[J]. Chin J Prevent Med, 2017, 51( 6): 457- 461. DOI: 10.3760/cma.j.issn.0253-9624.2017.06.001.徐爱强, 张丽. 中国病毒性肝炎血清流行病学调查的回顾与意义[J]. 中华预防医学杂志, 2017, 51( 6): 457- 461. DOI: 10.3760/cma.j.issn.0253-9624.2017.06.001. [5] YANG YQ, SHANG J, LU CZ, et al. Influencing factors for direct-acting antiviral therapy failure in treatment of hepatitis C[J]. J Clin Hepatol, 2022, 38( 5): 1059- 1063. DOI: 10.3969/j.issn.1001-5256.2022.05.016.杨宇晴, 尚佳, 卢诚震, 等. 直接抗病毒药物治疗丙型肝炎失败的影响因素分析[J]. 临床肝胆病杂志, 2022, 38( 5): 1059- 1063. DOI: 10.3969/j.issn.1001-5256.2022.05.016. [6] HE QF, HU R, ZENG YL, et al. Efficacy and safety of Sofosbuvir/Velpatasvir with or without ribavirin in treatment of patients with chronic hepatitis C virus genotype 3 infection: A real-world study[J/CD]. Chin J Liver Dis(Electronic Version), 2022, 14( 1): 6- 13. DOI: 10.3969/j.issn.1674-7380.2022.01.002.贺秋凤, 胡蓉, 曾义岚, 等. 索磷布韦/维帕他韦联合或不联合利巴韦林治疗基因3型慢性丙型肝炎病毒感染者的疗效及安全性: 一项真实世界研究[J/CD]. 中国肝脏病杂志(电子版), 2022, 14( 1): 6- 13. DOI: 10.3969/j.issn.1674-7380.2022.01.002. [7] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [8] GAYAM V, HOSSAIN MR, KHALID M, et al. Real-world clinical efficacy and tolerability of direct-acting antivirals in hepatitis C monoinfection compared to hepatitis C/human immunodeficiency virus coinfection in a community care setting[J]. Gut Liver, 2018, 12( 6): 694- 703. DOI: 10.5009/gnl18004. [9] MACHADO SM, VIGANI AG, LEITE AG, et al. Effectiveness of direct-acting antivirals for hepatitis C virus infection in hepatitis C/HIV coinfected individuals: A multicenter study[J]. Medicine, 2020, 99( 30): e21270. DOI: 10.1097/MD.0000000000021270. [10] TANG Q, WEI L, LIU XQ, et al. Sofosbuvir-based therapies achieved satisfactory virological response in Chinese individuals with genotypes 3 and 6 infections: A real-world experience[J]. Infect Drug Resist, 2021, 14: 2297- 2307. DOI: 10.2147/IDR.S312902. [11] ATSUKAWA M, TSUBOTA A, KONDO C, et al. Real-world clinical application of 12-week sofosbuvir/velpatasvir treatment for decompensated cirrhotic patients with genotype 1 and 2: A prospective, multicenter study[J]. Infect Dis Ther, 2020, 9( 4): 851- 866. DOI: 10.1007/s40121-020-00329-y. [12] MANGIA A, MILLIGAN S, KHALILI M, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts[J]. Liver Int, 2020, 40( 8): 1841- 1852. DOI: 10.1111/liv.14537. [13] JI FP, LI J, LIU L, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b[J]. J Gastroenterol Hepatol, 2021, 36( 3): 767- 774. DOI: 10.1111/jgh.15192. -



 PDF下载 ( 800 KB)
PDF下载 ( 800 KB)

 下载:
下载:


