血小板-白蛋白-胆红素指数(PALBI)联合AIMS65评分对肝硬化并发急性上消化道出血患者短期预后的预测价值
DOI: 10.12449/JCH240213
Value of platelet-albumin-bilirubin index combined with AIMS65 score in predicting the short-term prognosis of patients with liver cirrhosis and acute upper gastrointestinal bleeding
-
摘要:
目的 探讨血小板-白蛋白-胆红素指数(PALBI)联合AIMS65评分对肝硬化并发急性上消化道出血(AUGIB)患者入院后6周内再出血及死亡的预测价值。 方法 选取2021年2月—2022年10月在锦州医科大学附属第一医院住院治疗的肝硬化并发AUGIB患者238例,所有纳入患者均随访6周,根据预后情况分为死亡组(n=65)和生存组(n=173)、未再出血组(n=149)和再出血组(n=89)。收集患者的一般资料及实验室指标(血常规,肝、肾功能及凝血指标等),计算入院时的PALBI评分、AIMS65评分、Child-Turcotte-Pugh(CTP)评分、终末期肝病模型(MELD)评分。计量资料两组间比较采用成组t检验或Mann-Whitney U检验;计数资料两组间比较采用χ2检验。采用多因素Logistic回归模型分析肝硬化并发AUGIB患者入院治疗后6周内死亡或再出血的危险因素。通过受试者工作特征曲线(ROC曲线)及曲线下面积(AUC)评估各评分系统的预测效能;AUC的比较采用DeLong检验。 结果 死亡组和生存组患者比较,呕血、既往有静脉曲张病史、Alb、TBil、INR、Cr、PT、收缩压、PALBI评分、AIMS65评分、CTP评分和MELD评分差异均有统计学意义(P值均<0.05);多因素Logistic回归分析结果显示,呕血(OR=4.34,95%CI:1.88~10.05,P<0.001)、既往有静脉曲张病史(OR=3.51,95%CI:1.37~8.98,P=0.009)、PALBI评分(OR=4.49,95%CI:1.48~13.64,P=0.008)及AIMS65评分(OR=3.85,95%CI:2.35~6.30,P<0.001)是患者死亡的独立危险因素;各评分预测生存情况的ROC曲线结果显示,CTP评分、MELD评分、PALBI评分、AIMS65评分、PALBI联合AIMS65评分的AUC分别为0.758、0.798、0.789、0.870、0.888,其中PALBI联合AIMS65评分的AUC明显高于4种评分单独预测的AUC(P值均<0.05)。再出血组和未再出血组患者比较,呕血、糖尿病病史、Alb、TBil、INR、Cr、PT、PALBI评分、AIMS65评分、CTP评分和MELD评分差异均有统计学意义(P值均<0.05);多因素Logistic回归分析结果显示,PALBI评分(OR=2.41,95%CI:1.17~4.95,P=0.017)和AIMS65评分(OR=1.58,95%CI:1.17~2.15,P=0.003)是患者再出血的独立危险因素;各评分预测再出血的ROC曲线结果显示,CTP评分、MELD评分、PALBI评分、AIMS65评分、PALBI联合AIMS65评分的AUC分别为0.680、0.719、0.709、0.711、0.741,PALBI联合AIMS65评分的AUC最大(P值均<0.05),但特异度较低。 结论 PALBI评分联合AIMS65评分对肝硬化并发AUGIB患者入院后6周内的死亡具有一定的预测价值,优于CTP评分及MELD评分单独检测;对6周内再出血预测价值较低,准确性一般。 Abstract:Objective To investigate the value of platelet-albumin-bilirubin index (PALBI) combined with AIMS65 score in predicting rebleeding and death within 6 weeks after admission in patients with liver cirrhosis and acute upper gastrointestinal bleeding (AUGIB). Methods A retrospective study was conducted for 238 patients with liver cirrhosis and AUGIB who were hospitalized in The First Affiliated Hospital of Jinzhou Medical University from February 2021 to October 2022, and all patients were followed up for 6 weeks. According to the prognosis, they were divided into death group with 65 patients and survival group with 173 patients, and according to the presence or absence of rebleeding, they were divided into non-rebleeding group with 149 patients and rebleeding group with 89 patients. General data and laboratory markers (including blood routine, liver/renal function, and coagulation), and PALBI, AIMS65 score, Child-Turcotte-Pugh (CTP) score, and Model for End-stage Liver Disease (MELD) score were calculated on admission. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. A multivariate logistic regression model analysis was used to investigate the risk factors for death or rebleeding within 6 weeks after admission in patients with liver cirrhosis and AUGIB. The receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to investigate the predictive efficacy of each scoring system, and the DeLong test was used for comparison of AUC. Results There were significant differences between the death group and the survival group in hematemesis, past history of varices, albumin (Alb), total bilirubin (TBil), international normalized ratio (INR), creatinine (Cr), prothrombin time (PT), systolic blood pressure, PALBI, AIMS65 score, CTP score, and MELD score (all P<0.05). The multivariate logistic regression analysis showed that hematemesis (odds ratio [OR]=4.34, 95% confidence interval [CI]: 1.88 — 10.05, P<0.001), past history of varices (OR=3.51, 95%CI: 1.37 — 8.98, P=0.009), PALBI (OR=4.49, 95%CI: 1.48 — 13.64, P=0.008), and AIMS65 score (OR=3.85, 95%CI: 2.35 — 6.30, P<0.001) were independent risk factors for death. The ROC curve analysis of each scoring system in predicting survival showed that CTP score, MELD score, PALBI, AIMS65 score, and PALBI combined with AIMS65 score had an AUC of 0.758, 0.798, 0.789, 0.870, and 0.888, respectively, suggesting that PALBI combined with AIMS65 score had a significantly larger AUC than the four scoring systems used alone (all P<0.05). There were significant differences between the rebleeding group and the non-rebleeding group in hematemesis, history of diabetes, Alb, TBil, INR, Cr, PT, PALBI, AIMS65 score, CTP score, and MELD score (all P<0.05). The multivariate logistic regression analysis showed that PALBI (OR=2.41, 95%CI: 1.17 — 4.95, P=0.017) and AIMS65 score (OR=1.58, 95%CI: 1.17 — 2.15, P=0.003) were independent risk factors for rebleeding. The ROC curve analysis of each scoring system in predicting rebleeding showed that CTP score, MELD score, PALBI, AIMS65 score, and PALBI combined with AIMS65 score had an AUC of 0.680, 0.719, 0.709, 0.711, and 0.741, respectively, suggesting that PALBI combined with AIMS65 score had the largest AUC (all P<0.05), but with a relatively low specificity. Conclusion PALBI combined with AIMS65 score has a certain value in predicting death within 6 weeks after admission in patients with liver cirrhosis and AUGIB, with a better value than CTP score and MELD score alone. PALBI combined with AIMS65 score has a relatively low value in predicting rebleeding within 6 weeks, with an acceptable accuracy. -
Key words:
- Liver Cirrhosis /
- Acute Upper Gastrointestinal Bleeding /
- PALBI Score /
- AIMS65 Score /
- Prognosis
-
表 1 CTP评分
Table 1. CTP rating scale
指标 1分 2分 3分 腹水 无 轻度 中、重度 肝性脑病 无 1~2 3~4 Alb(g/L) >35 28~35 <28 TBil(µmol/L) <34 34~51 >51 PT(s) <4 4~6 >6 注:A级,5~6分;B级,7~9分;C级,10分及以上。 表 2 所有纳入患者的基线特征
Table 2. Baseline characteristics of all included patients
指标 数值 男/女(例) 162/76 呕血[例(%)] 103(43.28) 黑便[例(%)] 177(74.37) 既往有静脉曲张病史[例(%)] 69(28.99) 腹水[例(%)] 54(22.69) 肝性脑病[例(%)] 50(21.01) 高血压[例(%)] 42(17.65) 糖尿病[例(%)] 52(21.85) 吸烟史[例(%)] 99(41.60) 饮酒史[例(%)] 108(45.38) 年龄(岁) 59.68±11.31 收缩压(mmHg) 115.00(104.00~132.75) PLT(×109/L) 102.60(65.58~158.48) Hb(g/L) 73.45(56.80~95.28) WBC(×109/L) 6.60(4.31~8.96) ALT(U/L) 23.00(16.00~36.00) AST(U/L) 32.00(22.00~57.50) ALP(U/L) 89.00(62.00~130.00) GGT(U/L) 48.50(23.25~133.25) Alb(g/L) 28.90(24.48~33.00) TBil(µmol/L) 26.10(16.70~46.98) Cr(µmol/L) 69.85(55.10~96.65) INR 1.42(1.29~1.67) PT(s) 15.15(13.72~17.98) CTP评分 7.00(6.00~9.00) MELD评分 7.75(3.66~13.06) AIMS65评分 2.00(1.00~3.00) PALBI评分 -1.87±0.49 表 3 生存组和死亡组一般资料及生化指标比较
Table 3. Comparison of general data and biochemical indexes between survival group and death group
指标 生存组(n=173) 死亡组(n=65) 统计值 P值 男/女(例) 119/54 43/22 χ2=0.151 0.698 年龄(岁) 59.23±11.57 60.89±10.60 t=-1.009 0.314 呕血[例(%)] 54(31.21) 49(75.38) χ2=37.552 <0.001 黑便[例(%)] 134(77.46) 43(66.15) χ2=3.167 0.075 既往有静脉曲张[例(%)] 44(25.43) 25(38.46) χ2=3.895 0.048 腹水[例(%)] 40(23.12) 14(21.54) χ2=0.067 0.795 肝性脑病[例(%)] 32(18.50) 18(27.69) χ2=2.407 0.121 高血压[例(%)] 29(16.76) 13(20.00) χ2=0.341 0.559 糖尿病[例(%)] 43(24.86) 9(13.85) χ2=3.354 0.067 吸烟史[例(%)] 77(44.51) 22(33.85) χ2=2.211 0.137 饮酒史[例(%)] 80(46.24) 28(43.08) χ2=0.191 0.662 肝硬化病因[例(%)] χ2=15.073 0.237 HBV 61(35.26) 17(26.15) HCV 11(6.36) 4(6.15) 酒精性 67(38.73) 20(30.77) 原发胆汁性 13(7.51) 1(1.54) 自身免疫性 3(1.73) 1(1.54) 隐源性 30(17.34) 23(35.38) 病毒性合并酒精性 67(38.73) 1(1.54) 收缩压(mmHg) 119.00(107.00~133.00) 107.00(86.00~127.00) Z=-3.747 <0.001 PLT(×109/L) 99.00(64.60~143.20) 118.20(69.60~198.60) Z=-1.913 0.056 Hb(g/L) 71.90(56.70~95.80) 78.10(56.80~94.00) Z=-0.726 0.468 WBC(×109/L) 6.41(4.01~8.76) 7.34(5.41~9.22) Z=-1.819 0.069 ALT(U/L) 23.00(14.00~36.00) 23.00(17.00~34.00) Z=-0.550 0.583 AST(U/L) 32.00(21.00~55.00) 36.00(25.00~72.00) Z=-1.513 0.130 ALP(U/L) 87.00(61.00~125.00) 94.00(65.00~156.00) Z=-1.579 0.114 GGT(U/L) 45.00(22.00~112.00) 65.00(28.00~159.00) Z=-1.598 0.110 Alb(g/L) 30.00(26.00~34.00) 25.00(22.00~28.50) Z=-5.009 <0.001 TBil(µmol/L) 22.70(15.40~36.10) 35.60(26.00~92.00) Z=-4.912 <0.001 Cr(umol/L) 64.10(54.40~83.30) 86.30(70.10~174.00) Z=-4.645 <0.001 INR 1.36(1.28~1.57) 1.64(1.41~2.03) Z=-4.394 <0.001 PT(s) 14.60(13.70~16.80) 17.30(14.40~21.90) Z=-4.039 <0.001 CTP评分 7.00(6.00~8.00) 9.00(7.00~11.00) Z=-6.228 <0.001 MELD评分 6.12(2.33~9.95) 13.51(9.17~22.69) Z=-7.073 <0.001 AIMS65评分 1.00(0.00~2.00) 3.00(2.00~3.00) Z=-9.062 <0.001 PALBI评分 -2.00±0.47 -1.52±0.38 t=-7.398 <0.001 表 4 未再出血组和再出血组一般资料及生化指标比较
Table 4. Comparison of general data and biochemical indexes between non-rebleeding group and rebleeding group
指标 未再出血组(n=149) 再出血组(n=89) 统计值 P值 男/女(例) 102/47 60/29 χ2=0.028 0.868 年龄(岁) 59.36±11.80 60.22±10.50 t=-0.568 0.571 呕血[例(%)] 49(32.89) 54(60.67) χ2=17.527 <0.001 黑便[例(%)] 111(74.50) 66(74.16) χ2=0.003 0.954 既往有静脉曲张[例(%)] 37(24.83) 32(35.96) χ2=3.348 0.067 腹水[例(%)] 31(20.81) 23(25.84) χ2=0.806 0.369 肝性脑病[例(%)] 30(20.13) 20(22.47) χ2=0.183 0.668 高血压[例(%)] 25(16.78) 17(19.10) χ2=0.207 0.649 糖尿病[例(%)] 39(26.17) 13(14.61) χ2=4.367 0.037 吸烟史[例(%)] 68(45.64) 31(34.83) χ2=2.678 0.102 饮酒史[例(%)] 69(46.31) 39(43.82) χ2=0.139 0.709 肝硬化病因[例(%)] χ2=10.853 0.542 HBV 52(34.90) 26(29.21) HCV 9(6.04) 6(6.74) 酒精性 56(37.58) 31(34.83) 原发胆汁性 12(8.05) 2(2.25) 自身免疫性 2(1.34) 2(2.25) 隐源性 29(19.46) 24(26.97) 病毒性合并酒精性 9(6.04) 2(2.25) 收缩压(mmHg) 117.00(106.00~132.00) 110.00(94.00~137.00) Z=-1.174 0.241 PLT(×109/L) 98.90(65.50~139.20) 115.80(65.80~184.30) Z=-1.585 0.113 Hb(g/L) 70.70(54.90~94.60) 76.20(61.30~96.40) Z=-1.446 0.148 WBC(×109/L) 6.56(3.97~8.62) 7.08(5.05~9.44) Z=-1.654 0.098 ALT(U/L) 22.00(14.00~36.00) 23.00(17.00~37.00) Z=-0.976 0.329 AST(U/L) 30.00(21.00~54.00) 35.00(25.00~80.00) Z=-1.943 0.052 ALP(U/L) 85.00(61.00~129.00) 93.00(65.00~140.00) Z=-1.135 0.256 GGT(U/L) 45.00(22.00~108.00) 64.00(27.00~158.00) Z=-1.676 0.094 Alb(g/L) 29.00(26.00~33.00) 26.10(23.00~31.00) Z=-3.232 0.001 TBil(µmol/L) 22.90(15.40~34.00) 32.70(19.80~86.90) Z=-3.966 <0.001 Cr(µmol/L) 63.90(54.20~81.50) 84.60(61.60~155.00) Z=-4.584 <0.001 INR 1.37(1.29~1.58) 1.53(1.28~1.81) Z=-2.606 0.009 PT(s) 14.60(13.80~17.00) 16.00(13.70~19.60) Z=-2.482 0.013 CTP评分 7.00(6.00~8.00) 9.00(7.00~10.00) Z=-4.717 <0.001 MELD评分 6.35(2.31~9.44) 11.24(5.77~21.07) Z=-5.661 <0.001 AIMS65评分 1.00(1.00~2.00) 2.00(1.00~3.00) Z=-5.608 <0.001 PALBI评分 -2.00±0.46 -1.65±0.47 t=-5.629 <0.001 表 5 生存组及死亡组中的多因素分析
Table 5. Multivariate analysis of survival group and death group
变量 B值 SE Wald P值 OR 95%CI 呕血 1.469 0.428 11.782 <0.001 4.34 1.88~10.05 既往有静脉曲张 1.255 0.479 6.864 0.009 3.51 1.37~8.98 PALBI评分 1.501 0.568 6.992 0.008 4.49 1.48~13.64 AIMS65评分 1.348 0.251 28.793 <0.001 3.85 2.35~6.30 表 6 未再出血组及再出血组中的多因素分析
Table 6. Multivariate analysis of non-rebleeding group and rebleeding group
变量 B值 SE Wald P值 OR 95%CI 呕血 0.609 0.315 3.732 0.053 1.84 0.99~3.41 糖尿病病史 -0.550 0.392 1.969 0.161 0.58 0.27~1.24 PALBI评分 0.880 0.368 5.728 0.017 2.41 1.17~4.95 AIMS65评分 0.458 0.156 8.635 0.003 1.58 1.17~2.15 表 7 四种评分系统单独及联合预测6周内死亡的ROC曲线结果
Table 7. ROC curve results of death within 6 weeks predicted by four scoring systems alone and combined detection
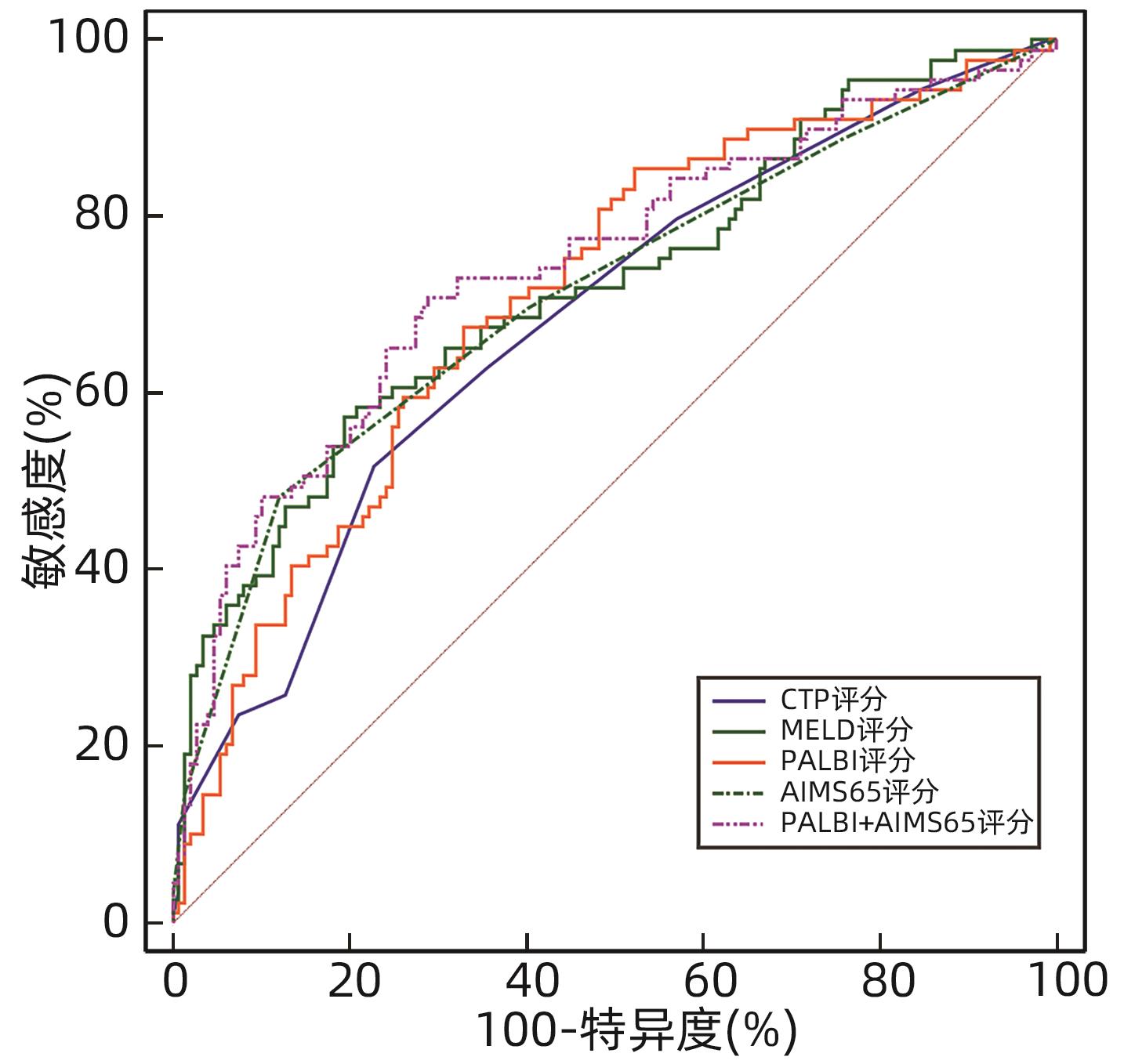
评分系统 AUC(95%CI) 敏感度(%) 特异度(%) 最佳截断值 P值 CTP评分 0.758(0.699~0.811)1) 64.62 78.03 8.00 <0.001 MELD评分 0.798(0.741~0.847)1) 80.00 69.94 8.53 <0.001 PALBI评分 0.789(0.732~0.839)1) 78.46 66.47 -1.83 <0.001 AIMS65评分 0.870(0.821~0.910)1) 69.23 90.75 2.00 <0.001 PALBI+AIMS65 0.888(0.841~0.925) 86.15 79.19 <0.001 注:与PALBI+AIMS65比较,1)P<0.05。 表 8 四种评分系统单独及联合预测6周内再出血的ROC曲线结果
Table 8. ROC curve results of rebleeding within 6 weeks predicted by four scoring systems alone and combined detection
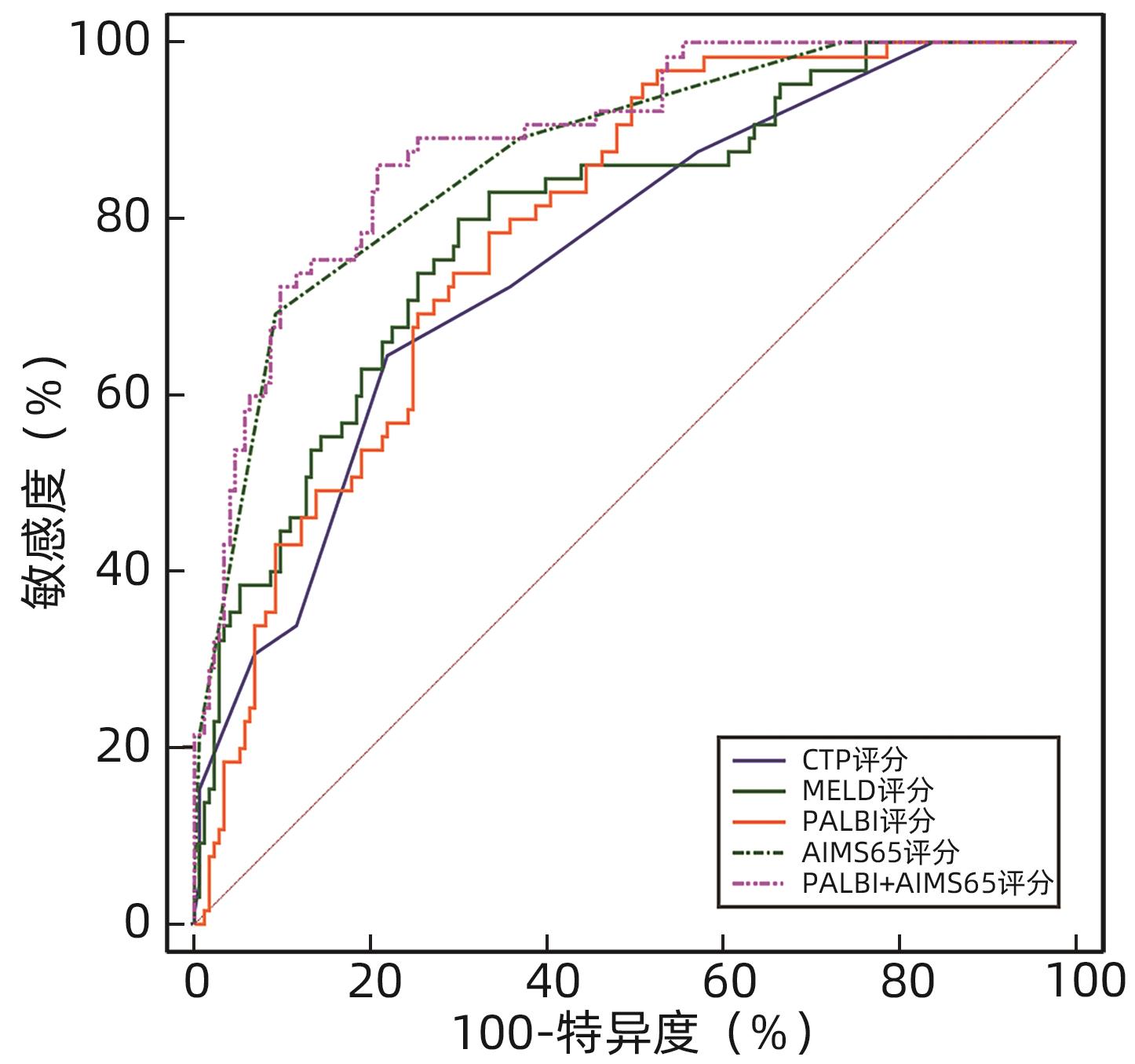
评分系统 AUC(95%CI) 敏感度(%) 特异度(%) 最佳截断值 P值 CTP评分 0.680(0.617~0.739)1) 51.69 77.18 8.00 <0.001 MELD评分 0.719(0.658~0.776)1) 57.30 80.54 10.55 <0.001 PALBI评分 0.709(0.647~0.766)1) 67.42 67.11 -1.83 <0.001 AIMS65评分 0.711(0.649~0.768)1) 48.31 87.92 2.00 <0.001 PALBI+AIMS65 0.741(0.681~0.796) 70.79 71.14 <0.001 注:与PALBI+AIMS65比较,1)P<0.05。 -
[1] CHANDNA S, ZARATE ER, GALLEGOS-OROZCO JF. Management of decompensated cirrhosis and associated syndromes[J]. Surg Clin North Am, 2022, 102( 1): 117- 137. DOI: 10.1016/j.suc.2021.09.005. [2] WU X, ZHANG XJ, XUE Y, et al. Changes and clinical significance of blood antithrombin-III and D-dimer levels in liver cirrhosis patients with gastrointestinal bleeding[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 56- 61. DOI: 10.3969/j.issn.1674-7380.2023.01.009.武幸, 张秀军, 薛源, 等. 肝硬化消化道出血患者血抗凝血酶Ⅲ和D-二聚体水平变化及临床意义[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 1): 56- 61. DOI: 10.3969/j.issn.1674-7380.2023.01.009. [3] ZHANG K, LIU JL, LIU YL, et al. Clinical effect of transjugular intrahepatic portosystemic shunt guided by the three-dimensional model constructed using thin-slice CT scan data in treatment of cirrhotic portal hypertension with gastrointestinal bleeding[J]. Clin J Med Offic, 2023, 51( 6): 655- 656, 660. DOI: 10.16680/j.1671-3826.2023.06.28.张凯, 刘晶磊, 刘燚隆, 等. 应用CT薄层扫描数据电脑构建3D模型指导经颈静脉肝内门体分流术治疗肝硬化门脉高压合并消化道出血临床效果观察[J]. 临床军医杂志, 2023, 51( 6): 655- 656, 660. DOI: 10.16680/j.1671-3826.2023.06.28. [4] JAKAB SS, GARCIA-TSAO G. Evaluation and management of esophageal and gastric varices in patients with cirrhosis[J]. Clin Liver Dis, 2020, 24( 3): 335- 350. DOI: 10.1016/j.cld.2020.04.011. [5] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [6] OIKONOMOU T, GOULIS L, DOUMTSIS P, et al. ALBI and PALBI grades are associated with the outcome of patients with stable decompensated cirrhosis[J]. Ann Hepatol, 2019, 18( 1): 126- 136. DOI: 10.5604/01.3001.0012.7904. [7] ROBERTSON M, NG J, SHAWISH W ABU, et al. Risk stratification in acute variceal bleeding: Comparison of the AIMS65 score to established upper gastrointestinal bleeding and liver disease severity risk stratification scoring systems in predicting mortality and rebleeding[J]. Dig Endosc, 2020, 32( 5): 761- 768. DOI: 10.1111/den.13577. [8] FREEMAN R. The new liver allocation system: Moving toward evidence-based transplantation policy[J]. Liver Transplant, 2002, 8( 9): 851- 858. DOI: 10.1053/jlts.2002.35927. [9] CHILD CG, TURCOTTE JG. Surgery and portal hypertension[J]. Major Probl Clin Surg, 1964, 1: 1- 85. [10] GARCIA-TSAO G, ABRALDES JG, BERZIGOTTI A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases[J]. Hepatology, 2017, 65( 1): 310- 335. DOI: 10.1002/hep.28906. [11] Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension(2019 edition)[J]. Chin J Pract Surg, 2019, 39( 12): 1241- 1247. DOI: 10.19538/j.cjps.issn1005-2208.2019.12.01.中华医学会外科学分会脾及门静脉高压外科学组. 肝硬化门静脉高压症食管、胃底静脉曲张破裂出血诊治专家共识(2019版)[J]. 中国实用外科杂志, 2019, 39( 12): 1241- 1247. DOI: 10.19538/j.cjps.issn1005-2208.2019.12.01. [12] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Endoscopy, Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2016, 32( 2): 203- 219. DOI: 10.3969/j.issn.1001-5256.2016.02.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2016, 32( 2): 203- 219. DOI: 10.3969/j.issn.1001-5256.2016.02.002. [13] Chinese Society of Hepatology, Chinese Society of Gastroenterology, and Chinese Society of Digestive Endoscopology of Chinese Medical Association. Guidelines on the management of esophagogastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2023, 39( 3): 527- 538.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会消化内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2023, 39( 3): 527- 538. [14] TANDON P, BISHAY K, FISHER S, et al. Comparison of clinical outcomes between variceal and non-variceal gastrointestinal bleeding in patients with cirrhosis[J]. J Gastroenterol Hepatol, 2018, 33( 10): 1773- 1779. DOI: 10.1111/jgh.14147. [15] LANAS A, DUMONCEAU JM, HUNT RH, et al. Non-variceal upper gastrointestinal bleeding[J]. Nat Rev Dis Primers, 2018, 4: 18020. DOI: 10.1038/nrdp.2018.20. [16] LI YY, LI HY, ZHU Q, et al. Effect of acute upper gastrointestinal bleeding manifestations at admission on the in-hospital outcomes of liver cirrhosis: Hematemesis versus melena without hematemesis[J]. Eur J Gastroenterol Hepatol, 2019, 31( 11): 1334- 1341. DOI: 10.1097/MEG.0000000000001524. [17] MANDAL AK, PAUDEL MS, KC S, et al. Factors predicting mortality of acute variceal bleeding in liver cirrhosis[J]. JNMA J Nepal Med Assoc, 2018, 56( 209): 493- 496. [18] GARCIA-TSAO G, BOSCH J. Varices and variceal hemorrhage in cirrhosis: A new view of an old problem[J]. Clin Gastroenterol Hepatol, 2015, 13( 12): 2109- 2117. DOI: 10.1016/j.cgh.2015.07.012. [19] ROAYAIE S, JIBARA G, BERHANE S, et al. 851 PALBI-An objective score based on platelets, albumin bilirubin stratifies HCC patients undergoing resection& ablation better than Child’s classification[J]. Hepatology, 2015, 62: 624- 690. [20] ELSHAARAWY O, ALLAM N, ABDELSAMEEA E, et al. Platelet-albumin-bilirubin score- a predictor of outcome of acute variceal bleeding in patients with cirrhosis[J]. World J Hepatol, 2020, 12( 3): 99- 107. DOI: 10.4254/wjh.v12.i3.99. -



 PDF下载 ( 909 KB)
PDF下载 ( 909 KB)

 下载:
下载: