铁皮石斛叶发酵液对酒精性肝炎小鼠模型的影响及其作用机制
DOI: 10.12449/JCH240218
Effect of Dendrobium officinale leaf fermentation fluid on a mouse model of alcoholic hepatitis and its mechanism of action
-
摘要:
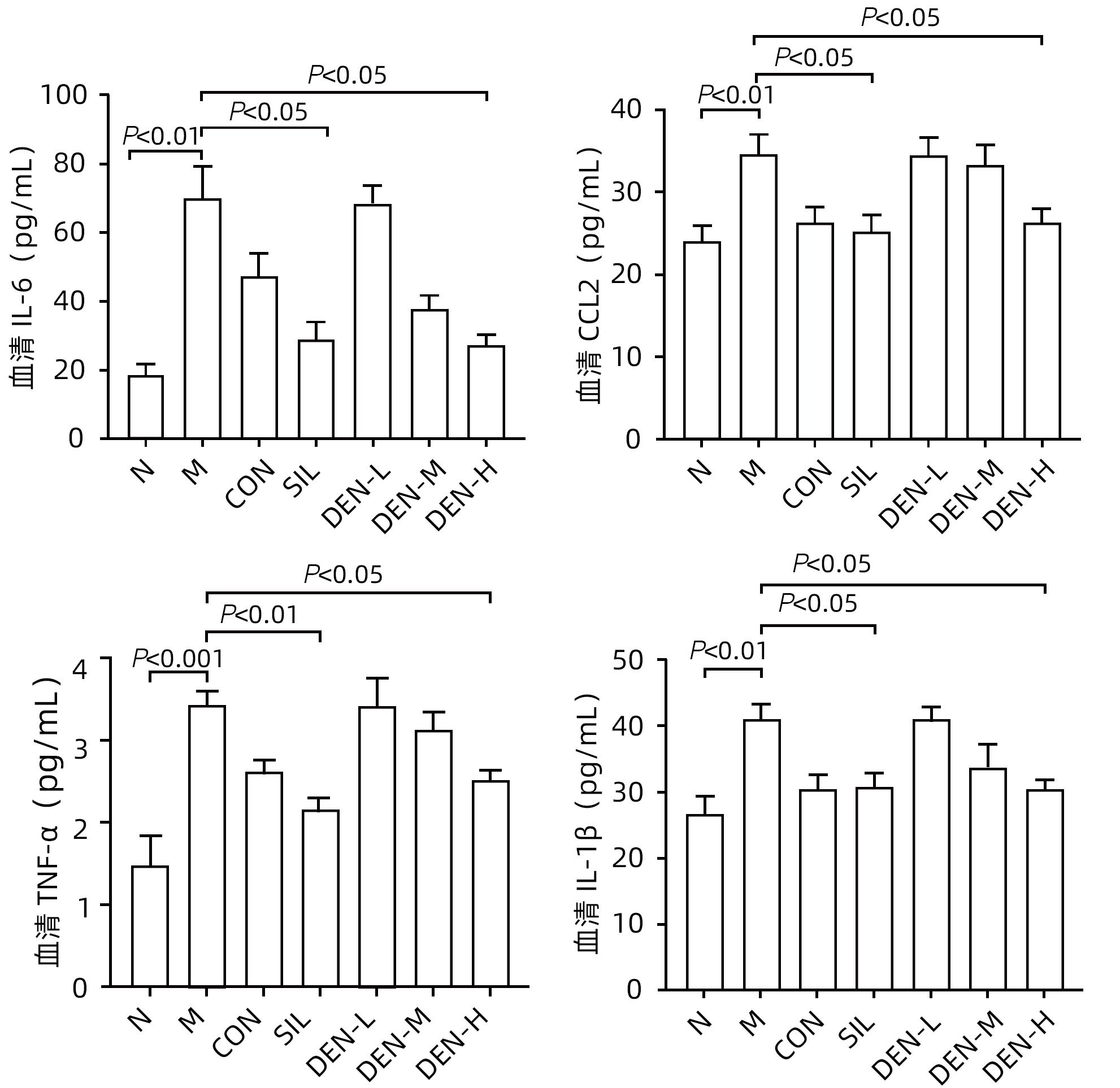
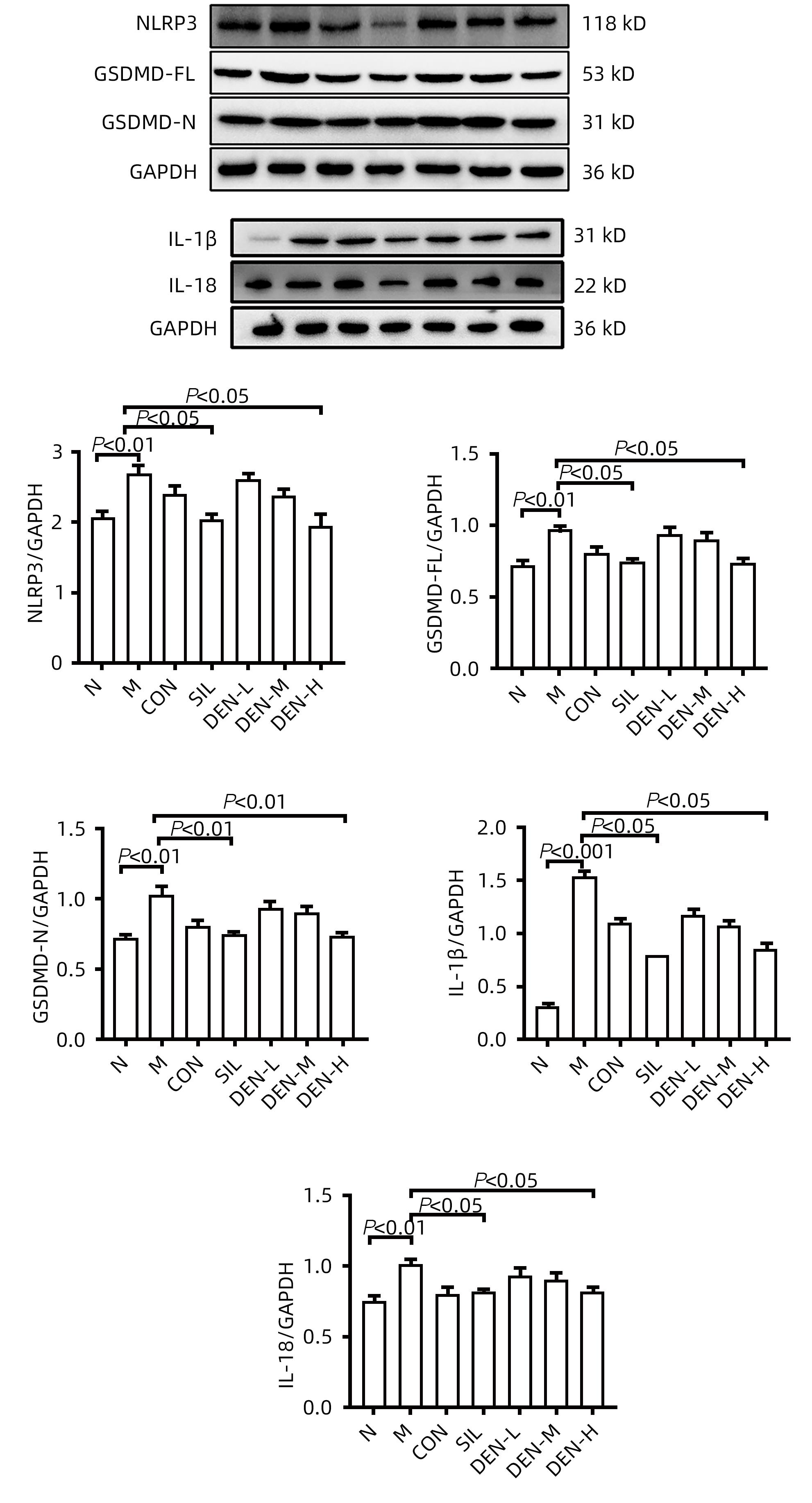
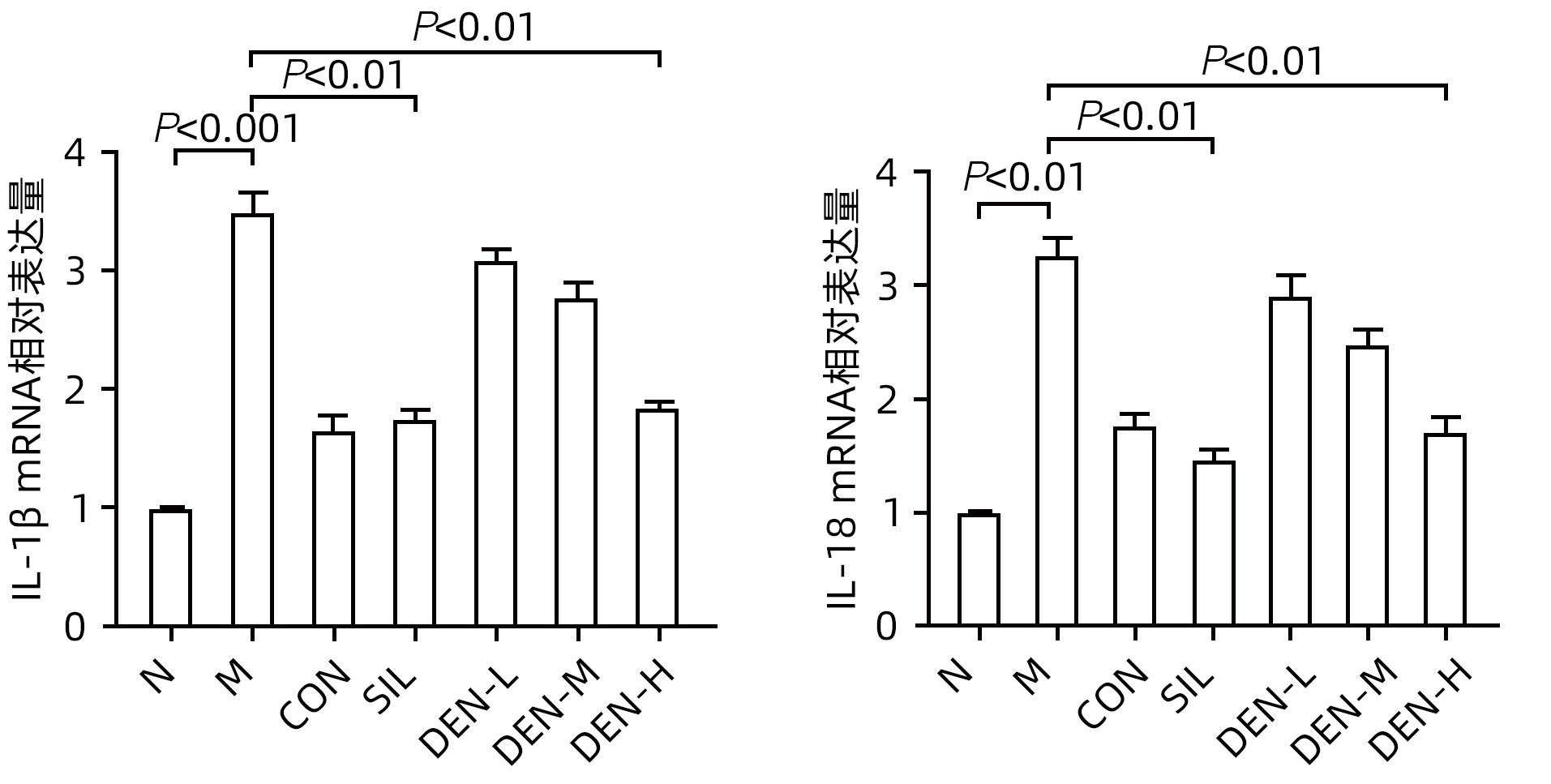
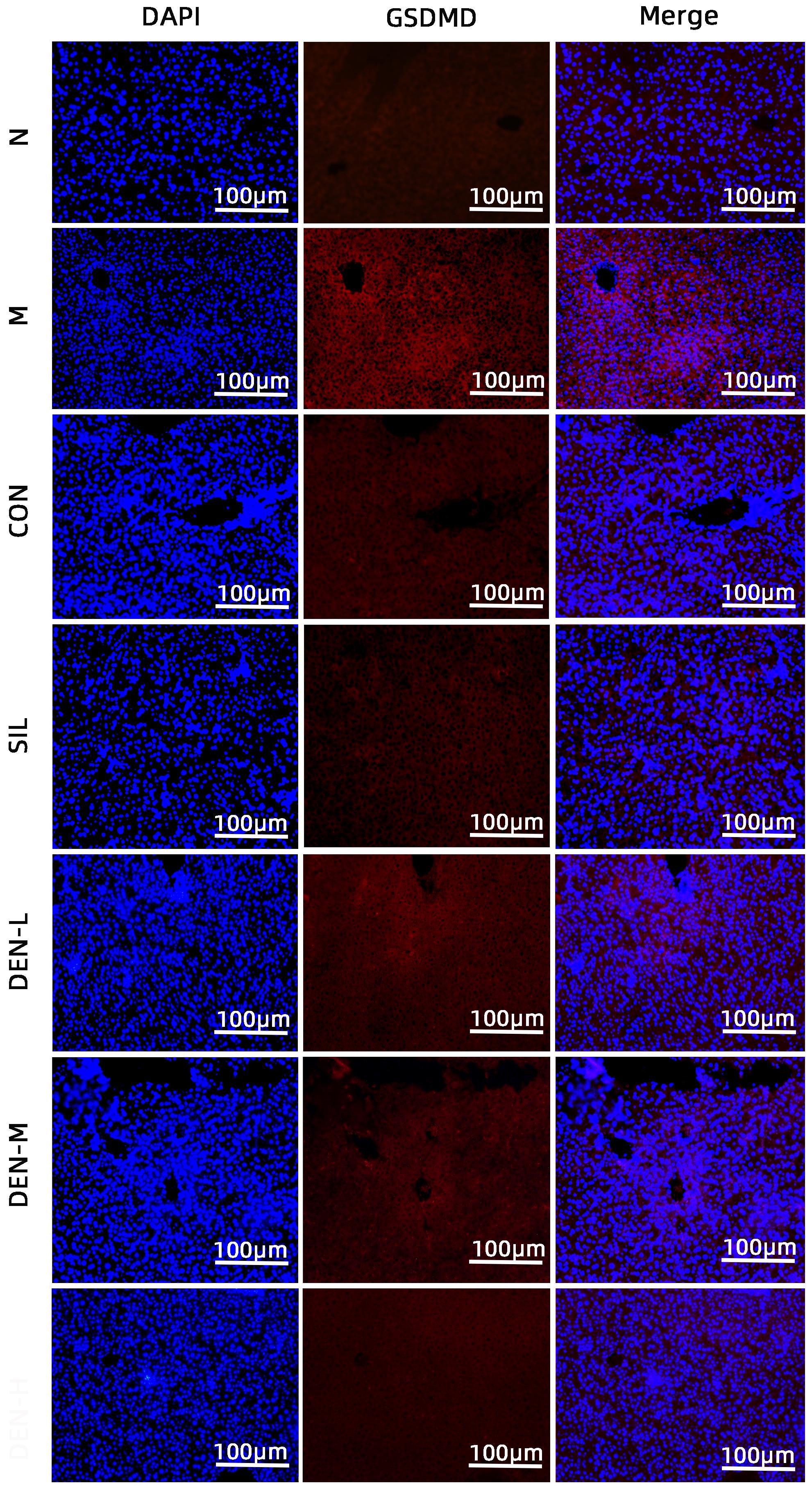
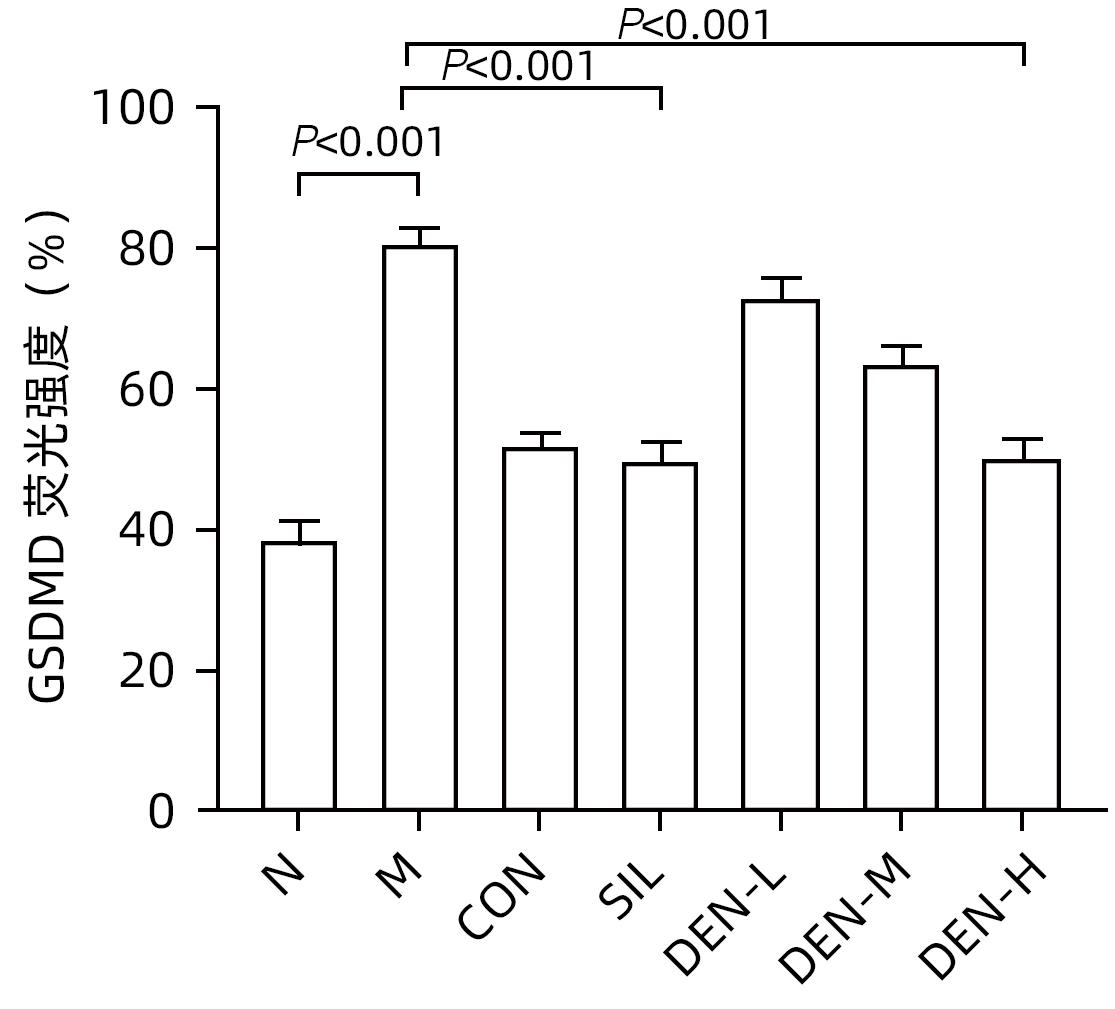
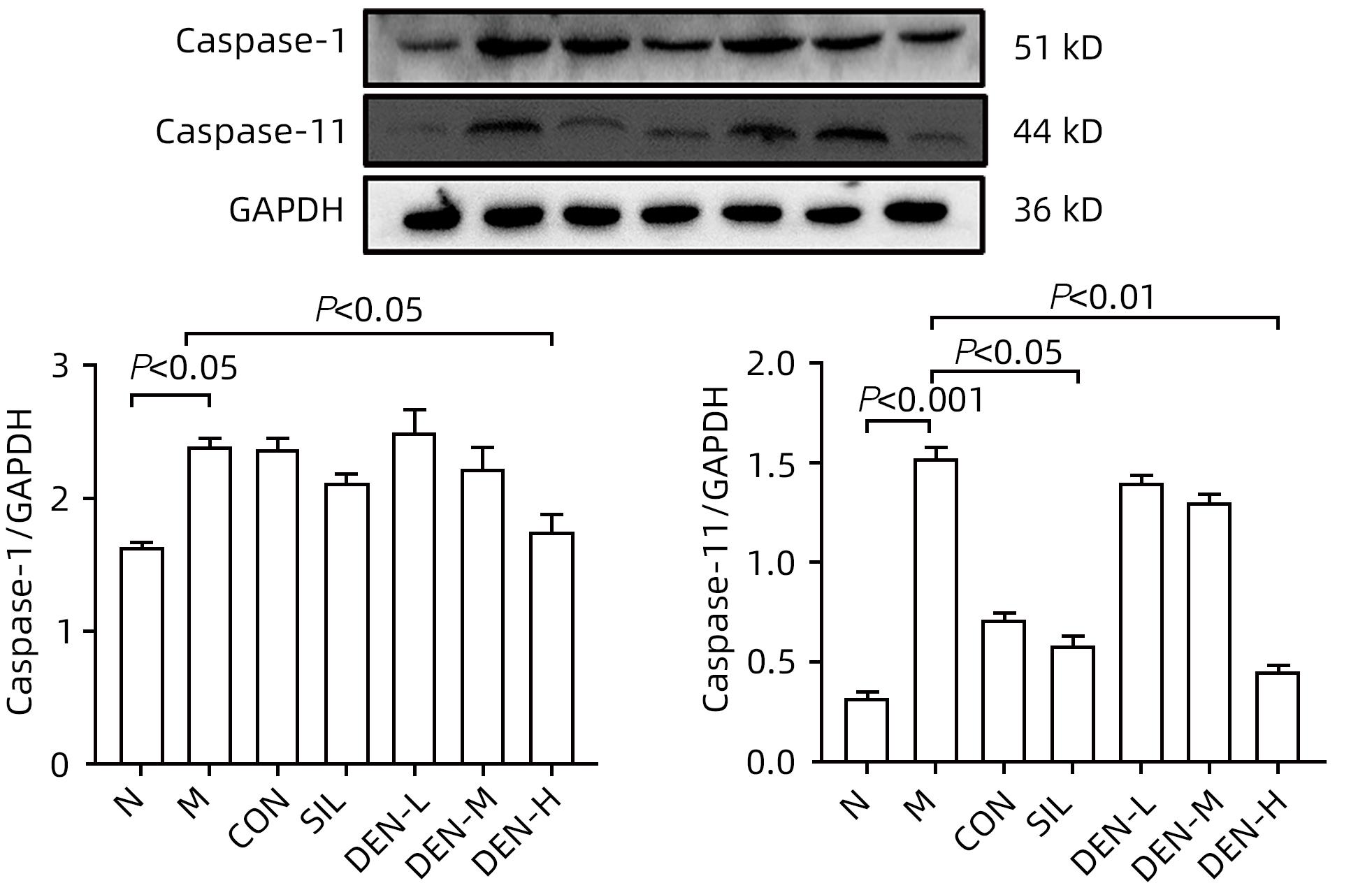
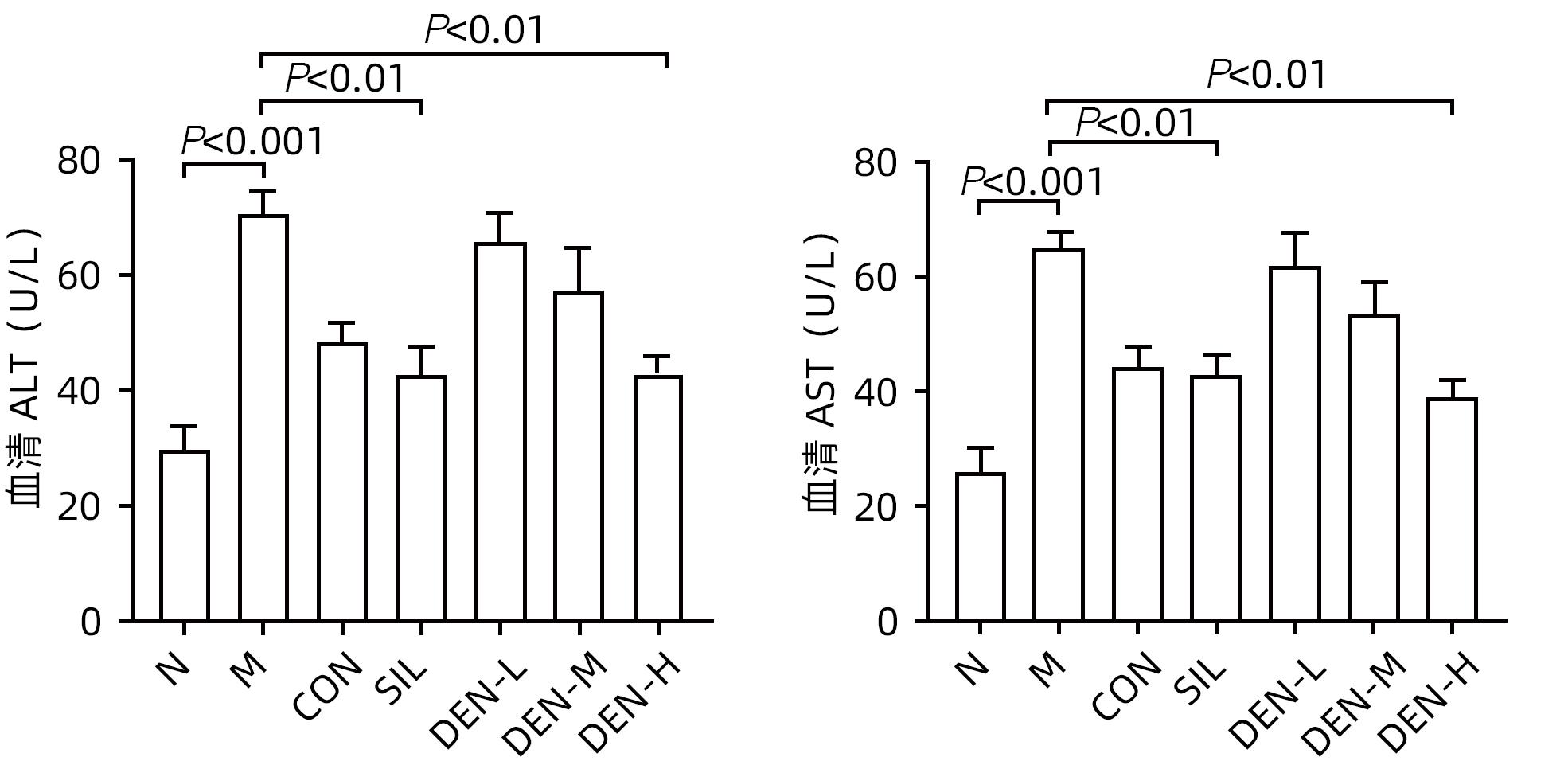
目的 探讨铁皮石斛叶发酵液对小鼠酒精性肝炎的干预机制。 方法 将70只健康雄性6~8周C57BL/6J小鼠随机分为正常组,模型组,液体饲料对照组,水飞蓟宾组,铁皮石斛叶发酵液低剂量组、中剂量组及高剂量组,共7组,每组各10只。正常组小鼠喂养普通饲料,其他各组小鼠采用Lieber-DeCarli经典法液体饲料饲养8周,诱导小鼠酒精性肝炎模型。造模期间,铁皮石斛叶发酵液低、中、高剂量组小鼠饮用威门石斛水,其余各组小鼠饮用纯净水;正常组、模型组、液体饲料对照组每日给予生理盐水灌胃,水飞蓟宾组给予水飞蓟宾0.25 mL/10 g,铁皮石斛叶发酵液低、中、高剂量组分别给予0.125 mL/10 g、0.250 mL/10 g、0.375 mL/10 g铁皮石斛叶发酵液灌胃,1次/d。第8周水合氯醛腹腔注射麻醉,眼球取血,分离血清采用生化方法检测AST、ALT水平;HE染色和油红O染色观察小鼠肝组织病理学以及脂质积累情况。Luminex多因子检测小鼠血清IL-6、IL-1β、TNF-α及CCL2水平;实时荧光定量PCR、Western Blot、免疫荧光方法检测小鼠肝组织中NLRP3、Caspase-1、Caspase-11、GSDMD、GSDMD-N端、IL-18和IL-1β的蛋白表达。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与正常组比较,模型组小鼠血清AST、ALT、IL-6、IL-1β、TNF-α和CCL2水平均显著升高(P值均<0.05)。与模型组相比,铁皮石斛叶发酵液高剂量组小鼠血清AST、ALT、IL-6、IL-1β、TNF-α和CCL2均明显降低(P值均<0.05)。HE染色结果显示,模型组小鼠肝小叶结构紊乱,有大量的脂肪变性空泡及细胞坏死;与模型组比较,铁皮石斛叶发酵液高剂量组小鼠肝组织病理损伤减轻,大部分肝小叶结构完整,仅少量炎性细胞浸润。油红O染色结果显示,模型组小鼠肝脏出现大脂滴和小脂滴积聚,肝脂肪含量显著增加;与模型组比较,铁皮石斛叶发酵液高剂量组小鼠肝组织脂肪变性明显减轻,多以小脂滴散在存在。肝组织免疫荧光结果显示,模型组小鼠肝细胞胞质中GSDMD阳性染色面积比较正常组显著增加(P<0.001);铁皮石斛叶发酵液高剂量组小鼠肝细胞胞质中GSDMD阳性染色面积比与模型组比较显著下降(P<0.001)。实时荧光定量PCR和Western Blot检测结果显示,与正常组比较,模型组小鼠肝组织中NLRP3、Caspase-1、Caspase-11、GSDMD、GSDMD-N端、IL-18和IL-1β蛋白表达水平显著增加(P值均<0.05);与模型组相比,铁皮石斛叶发酵液高剂量组NLRP3、Caspase-1、Caspase-11、GSDMD、GSDMD-N端、IL-18和IL-1β蛋白表达水平明显降低(P值均<0.05)。与模型组相比,铁皮石斛叶发酵液高剂量组Caspase-1和Caspase-11蛋白表达水平均明显降低(P值均<0.05),其中Caspase-1蛋白相对表达量为1.757,较模型组下降26.6%,Caspase-11蛋白相对表达量为0.455,较模型组下降70.3%;Caspase-11较Caspase-1下降幅度更大。 结论 铁皮石斛叶发酵液可减轻小鼠酒精性肝炎,其机制可能与抑制Caspase-11介导的非经典细胞焦亡通路有关。 -
关键词:
- 肝炎, 酒精性 /
- 铁皮石斛 /
- 细胞焦亡 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the intervention mechanism of Dendrobium officinale leaf fermentation fluid in mice with alcoholic hepatitis. Methods A total of 70 healthy male C57BL/6J mice, aged 6-8 weeks, were randomly divided into normal group, model group, liquid feed control group, silybin group, and low-, middle-, and high-dose Dendrobium officinale leaf fermentation fluid groups, with 10 mice in each group. The mice in the normal group were given normal diet, and those in the other groups were given Lieber-DeCarli classic liquid diet for 8 weeks to induce alcoholic hepatitis. During modeling, the mice in the low-, middle-, and high-dose Dendrobium officinale leaf fermentation fluid groups were given Dendrobium liquid manufactured by Warmen Pharmaceutical, and the mice in all the other groups were given pure water; the mice in the normal group, the model group, and the liquid feed control group were given normal saline by gavage, those in the silybin group were given silybin 0.25 mL/10 g by gavage, and those in the low-, middle-, and high-dose Dendrobium officinale leaf fermentation fluid groups were given Dendrobium officinale leaf fermentation fluid at a dose of 0.125 mL/10 g, 0.250 mL/10 g, and 0.375 mL/10 g, respectively, by gavage, once a day. At week 8, chloral hydrate was injected intraperitoneally for anesthesia, and blood samples were collected from the eyeball. After serum was separated, the biochemical method was used to measure the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT); HE staining and oil red staining were used to observe liver histopathology and lipid accumulation in mice; multiplex Luminex assay was used to measure the serum levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and CCL2; quantitative real-time PCR, Western blot, and immunofluorescence assay were used to measure the protein expression levels of NLRP3, caspase-1, caspase-11, gasdermin D (GSDMD), N-terminal gasdermin D (GSDMD-N) in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the normal group, the model group had significant increases in the serum levels of AST, ALT, IL-6, IL-1β, TNF-α, and CCL2 (all P<0.05), and compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had significant reductions in the serum levels of AST, ALT, IL-6, IL-1β, TNF-α, and CCL2 (all P<0.05). HE staining showed that the model group had disordered structure of hepatic lobules, with a large number of steatosis vacuoles and massive cell necrosis, and compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had alleviation of liver histopathological injury, intact structure of most hepatic lobules, and a small amount of inflammatory cell infiltration. Oil red staining showed that the model group had accumulation of large and small lipid droplets in the liver and a significant increase in liver fat content, and compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had significant alleviation of hepatic steatosis, with the presence of sporadic small lipid droplets. Immunofluorescence assay of liver tissue showed that compared with the normal group, the model group had a significant increase in the ratio of GSDMD-positive staining area in hepatocyte cytoplasm (P<0.001), and compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had a significant reduction in such ratio in hepatocyte cytoplasm (P<0.001). Quantitative real-time PCR showed that compared with the normal group, the model group had significant increases in the protein expression levels of NLRP3, caspase-1, caspase-11, GSDMD, GSDMD-N, interleukin-18 (IL-18), and IL-1β in liver tissue (all P<0.05), and compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had significant reductions in the protein expression levels of NLRP3, caspase-1, caspase-11, GSDMD, GSDMD-N, IL-18, and IL-1 (all P<0.05). Compared with the model group, the high-dose Dendrobium officinale leaf fermentation fluid group had significant reductions in the protein expression levels of caspase-1 and caspase-11 (both P<0.05), with a relative expression level of caspase-1 of 1.757 (reduced by 26.6% compared with the model group) and a relative expression level of caspase-11 of 0.455 (reduced by 70.3% compared with the model group), suggesting that caspase-11 showed a greater reduction than caspase-1. Conclusion Dendrobium officinale leaf fermentation fluid can alleviate alcoholic hepatitis in mice, possibly by inhibiting the non-classical cell pyroptosis pathway mediated by caspase-11. -
Key words:
- Hepatitis, Alcoholic /
- Dendrobii Officmalis Caulis /
- Pyroptosis /
- Mice, Inbred C57BL
-
表 1 Lieber-DeCarli液体饲料配方
Table 1. Lieber-DeCarli liquid feed formula
项目 Lieber-DeCarli酒精液体饲料 Lieber-DeCarli对照液体饲料 温水,40 ℃左右 约600 mL 约600 mL 主料 148.0 g 148.0 g 胆碱 0.53 g 0.53 g 维生素 2.50 g 2.50 g 温水,40 ℃左右 200 mL 95%或无水乙醇 含50 mL酒精 水 定容至1 000 mL 定容至1 000 mL 表 2 实时荧光定量PCR引物
Table 2. Real-time quantitative PCR primers
引物名称 引物序列(5′-3′) 扩增长度(bp) GAPDH 上游:AGGTCGGTGTGAACGGATTTG 21 下游:GGGGTCGTTGATGGCAACA 19 IL-18 上游:GTGAACCCCAGACCAGACTG 20 下游:CCTGGAACACGTTTCTGAAAGA 22 IL-1β 上游:GAAATGCCACCTTTTGACAGTG 22 下游:TGGATGCTCTCATCAGGACAG 21 -
[1] ALTAMIRANO J, BATALLER R. Alcoholic liver disease: Pathogenesis and new targets for therapy[J]. Nat Rev Gastroenterol Hepatol, 2011, 8( 9): 491- 501. DOI: 10.1038/nrgastro.2011.134. [2] SHI JJ, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526( 7575): 660- 665. DOI: 10.1038/nature15514. [3] LIU X, XIA SY, ZHANG ZB, et al. Channelling inflammation: Gasdermins in physiology and disease[J]. Nat Rev Drug Discov, 2021, 20( 5): 384- 405. DOI: 10.1038/s41573-021-00154-z. [4] AMBADE A, LOWE P, KODYS K, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice[J]. Hepatology, 2019, 69( 3): 1105- 1121. DOI: 10.1002/hep.30249. [5] FENG LJ, LIU KY, QUAN HQ. Study on main composition and content determination of Dendrobium candidum[J]. Farm Prod Process, 2023( 6): 85- 87. DOI: 10.16693/j.cnki.1671-9646(X).2023.03.049.冯柳娟, 刘凯月, 全海倩. 铁皮石斛主要成分及其含量检测方法的研究[J]. 农产品加工, 2023( 6): 85- 87. DOI: 10.16693/j.cnki.1671-9646(X).2023.03.049. [6] LIU YG, YANG LL, ZHANG Y, et al. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure[J]. J Ethnopharmacol, 2020, 248: 112308. DOI: 10.1016/j.jep.2019.112308. [7] CHU WH, WANG P, MA Z, et al. Ultrasonic treatment of Dendrobium officinale polysaccharide enhances antioxidant and anti-inflammatory activity in a mouse D-galactose-induced aging model[J]. Food Sci Nutr, 2022, 10( 8): 2620- 2630. DOI: 10.1002/fsn3.2867. [8] YANG K, ZHAN LH, LU TT, et al. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κB signaling pathway[J]. Cytokine, 2020, 130: 155058. DOI: 10.1016/j.cyto.2020.155058. [9] YUAN C, LIAN QH, NI BB, et al. Screening and bioinformatics analysis of key autophagy-related genes in alcoholic hepatitis[J]. Ogran Transplant, 2024, 15( 1): 90- 101.袁超, 练庆海, 尼贝贝, 等. 酒精性肝炎自噬关键基因的筛选及生物信息学分析[J]. 器官移植, 2024, 15( 1): 90- 101. [10] XU H, XIAO P, ZHANG F, et al. Epidemic characteristics of alcohol-related liver disease in Asia from 2000 to 2020: A systematic review and meta-analysis[J]. Liver Int, 2022, 42( 9): 1991- 1998. DOI: 10.1111/liv.15312. [11] SUNG H, KIM SW, HONG M, et al. Microbiota-based treatments in alcoholic liver disease[J]. World J Gastroenterol, 2016, 22( 29): 6673- 6682. DOI: 10.3748/wjg.v22.i29.6673. [12] YI YS. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses[J]. Immunology, 2017, 152( 2): 207- 217. DOI: 10.1111/imm.12787. [13] KHANOVA E, WU R, WANG W, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients[J]. Hepatology, 2018, 67( 5): 1737- 1753. DOI: 10.1002/hep.29645. [14] HEO MJ, KIM TH, YOU JS, et al. Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression[J]. Gut, 2019, 68( 4): 708- 720. DOI: 10.1136/gutjnl-2017-315123. [15] WU YL, HUANG SH, HE CM, et al. Dendrobium officinale flower extraction mitigates alcohol-induced liver injury in mice: Role of antisteatosis, antioxidative, and anti-inflammatory[J]. Evid Based Complement Alternat Med, 2020, 2020: 1421853. DOI: 10.1155/2020/1421853. [16] YUAN HQ, LIANG CY, LIANG J, et al. Protection of Dendrobium Officinale against acute alcoholic liver injury in mice[J]. J Jinan Univ Nat Sci Med Ed, 2016, 37( 5): 384- 388. DOI: 10.11778/j.jdxb.2016.05.005.袁慧琦, 梁楚燕, 梁健, 等. 铁皮石斛对小鼠急性酒精性肝损伤的保护作用[J]. 暨南大学学报(自然科学与医学版), 2016, 37( 5): 384- 388. DOI: 10.11778/j.jdxb.2016.05.005. -



 PDF下载 ( 2486 KB)
PDF下载 ( 2486 KB)

 下载:
下载: