线粒体自噬在肝脏相关疾病发生发展中的作用
DOI: 10.12449/JCH240232
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:潘萌负责设计论文框架,起草论文;史晓燕负责拟定写作思路,指导文章修改与定稿。
-
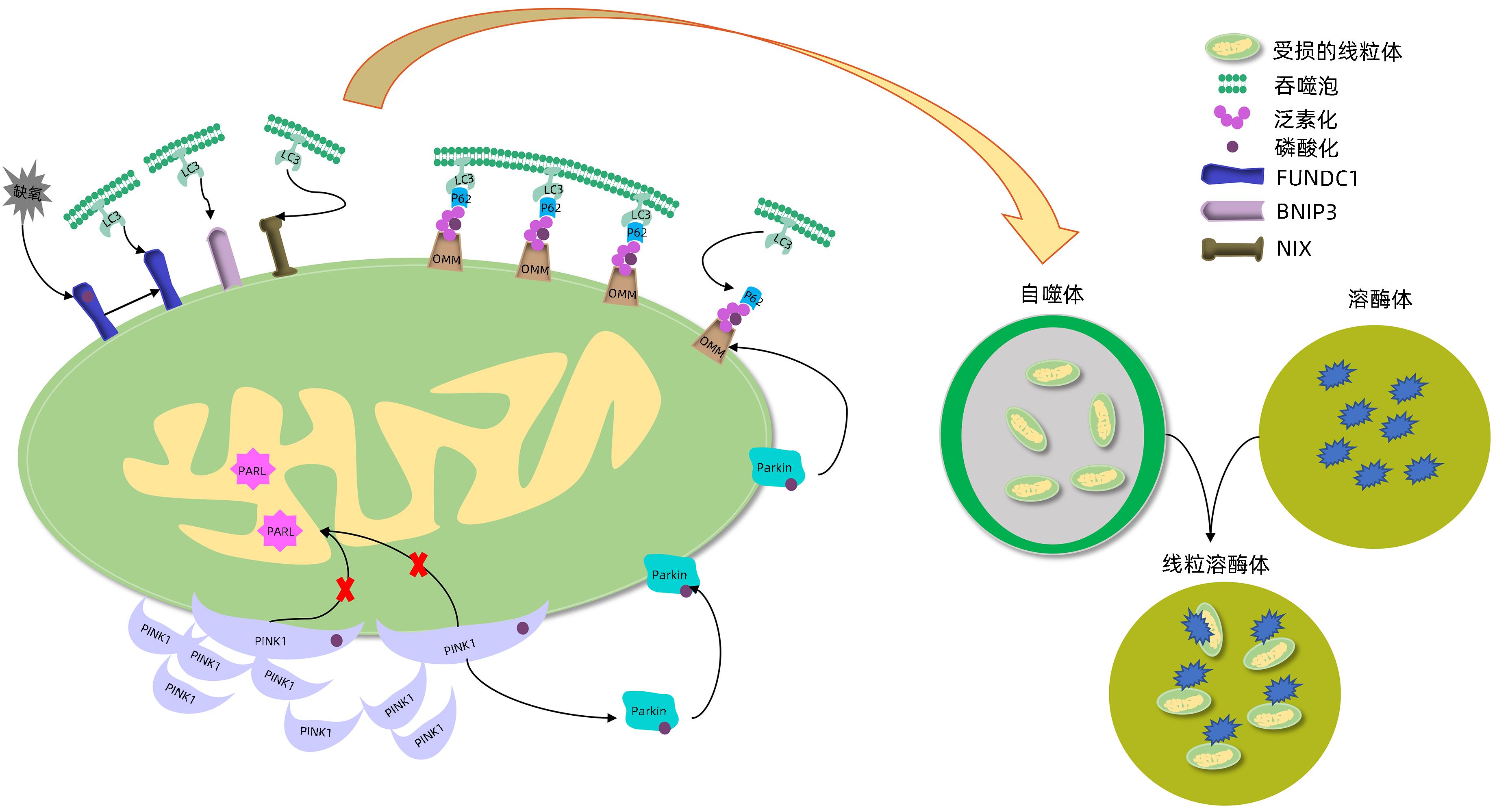
摘要: 线粒体自噬是细胞在营养缺乏或受到外界刺激时,通过对受损线粒体的特异性清除,来维持线粒体功能完整性和细胞稳态的选择性自噬。近年来,大量研究证明线粒体自噬功能失调与非酒精性脂肪性肝病、药物性肝损伤、病毒性肝炎和肝细胞癌等多种肝脏相关疾病的发生发展密切相关。本文通过对线粒体自噬调控肝脏相关疾病的具体机制进行总结,进一步阐述了线粒体自噬在肝脏相关疾病中的潜在治疗靶点,以期为肝病的临床治疗提供更为有效的策略。Abstract: Mitophagy is a type of selective autophagy during which cells specifically remove damaged mitochondria in response to nutrient deficiency or external stimulation and thus maintain the integrity of mitochondrial function and cellular homeostasis. In recent years, a large number of studies have shown that dysfunction of mitophagy is closely associated with the development and progression of various liver-related diseases such as nonalcoholic fatty liver disease, drug-related liver injury, viral hepatitis, and hepatocellular carcinoma. This article summarizes the specific mechanisms of mitophagy in regulating liver-related diseases and further elaborates on the potential therapeutic targets of mitophagy in liver-related diseases, in order to provide more effective therapeutic strategies for the clinical treatment of liver diseases.
-
Key words:
- Liver Diseases /
- Mitophagy /
- Pathologic Processes
-
[1] QIAN H, CHAO XJ, WILLIAMS J, et al. Autophagy in liver diseases: A review[J]. Mol Aspects Med, 2021, 82: 100973. DOI: 10.1016/j.mam.2021.100973. [2] GRATTAGLIANO I, RUSSMANN S, DIOGO C, et al. Mitochondria in chronic liver disease[J]. Curr Drug Targets, 2011, 12( 6): 879- 893. DOI: 10.2174/138945011795528877. [3] SORRENTINO V, MENZIES KJ, AUWERX J. Repairing mitochondrial dysfunction in disease[J]. Annu Rev Pharmacol Toxicol, 2018, 58: 353- 389. DOI: 10.1146/annurev-pharmtox-010716-104908. [4] GOIKOETXEA-USANDIZAGA N, SERRANO-MACIÁ M, DELGADO TC, et al. Mitochondrial bioenergetics boost macrophage activation, promoting liver regeneration in metabolically compromised animals[J]. Hepatology, 2022, 75( 3): 550- 566. DOI: 10.1002/hep.32149. [5] QIU YN, WANG GH, ZHOU F, et al. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy[J]. Ecotoxicol Environ Saf, 2019, 167: 178- 187. DOI: 10.1016/j.ecoenv.2018.08.050. [6] KE PY. Mitophagy in the pathogenesis of liver diseases[J]. Cells, 2020, 9( 4): 831. DOI: 10.3390/cells9040831. [7] GARZA-LOMBÓ C, PAPPA A, PANAYIOTIDIS MI, et al. Redox homeostasis, oxidative stress and mitophagy[J]. Mitochondrion, 2020, 51: 105- 117. DOI: 10.1016/j.mito.2020.01.002. [8] SUN K, JING XZ, GUO JC, et al. Mitophagy in degenerative joint diseases[J]. Autophagy, 2021, 17( 9): 2082- 2092. DOI: 10.1080/15548627.2020.1822097. [9] MA XW, MCKEEN T, ZHANG JH, et al. Role and mechanisms of mitophagy in liver diseases[J]. Cells, 2020, 9( 4): 837. DOI: 10.3390/cells9040837. [10] KLIONSKY DJ, EMR SD. Autophagy as a regulated pathway of cellular degradation[J]. Science, 2000, 290( 5497): 1717- 1721. DOI: 10.1126/science.290.5497.1717. [11] MIZUSHIMA N, LEVINE B, CUERVO AM, et al. Autophagy fights disease through cellular self-digestion[J]. Nature, 2008, 451( 7182): 1069- 1075. DOI: 10.1038/nature06639. [12] MIZUSHIMA N, KOMATSU M. Autophagy: Renovation of cells and tissues[J]. Cell, 2011, 147( 4): 728- 741. DOI: 10.1016/j.cell.2011.10.026. [13] YOULE RJ, NARENDRA DP. Mechanisms of mitophagy[J]. Nat Rev Mol Cell Biol, 2011, 12( 1): 9- 14. DOI: 10.1038/nrm3028. [14] LU YY, LI ZJ, ZHANG SQ, et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation[J]. Theranostics, 2023, 13( 2): 736- 766. DOI: 10.7150/thno.79876. [15] LIN JJ, CHEN K, CHEN WF, et al. Paradoxical mitophagy regulation by PINK1 and TUFm[J]. Mol Cell, 2020, 80( 4): 607- 620. DOI: 10.1016/j.molcel.2020.10.007. [16] YAN CJ, GONG LL, CHEN L, et al. PHB2(prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis[J]. Autophagy, 2020, 16( 3): 419- 434. DOI: 10.1080/15548627.2019.1628520. [17] KANE LA, LAZAROU M, FOGEL AI, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity[J]. J Cell Biol, 2014, 205( 2): 143- 153. DOI: 10.1083/jcb.201402104. [18] HARPER JW, ORDUREAU A, HEO JM. Building and decoding ubiquitin chains for mitophagy[J]. Nat Rev Mol Cell Biol, 2018, 19( 2): 93- 108. DOI: 10.1038/nrm.2017.129. [19] NGUYEN TN, PADMAN BS, LAZAROU M. Deciphering the molecular signals of PINK1/Parkin mitophagy[J]. Trends Cell Biol, 2016, 26( 10): 733- 744. DOI: 10.1016/j.tcb.2016.05.008. [20] ONISHI M, YAMANO K, SATO M, et al. Molecular mechanisms and physiological functions of mitophagy[J]. EMBO J, 2021, 40( 3): e104705. DOI: 10.15252/embj.2020104705. [21] HAMACHER-BRADY A, BRADY NR. Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy[J]. Cell Mol Life Sci, 2016, 73( 4): 775- 795. DOI: 10.1007/s00018-015-2087-8. [22] LIU L, FENG D, CHEN G, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells[J]. Nat Cell Biol, 2012, 14( 2): 177- 185. DOI: 10.1038/ncb2422. [23] FLORES-TORO JA, GO KL, LEEUWENBURGH C, et al. Autophagy in the liver: Cell's cannibalism and beyond[J]. Arch Pharm Res, 2016, 39( 8): 1050- 1061. DOI: 10.1007/s12272-016-0807-8. [24] ZHOU JH, ZHOU F, WANG WX, et al. Epidemiological features of NAFLD from 1999 to 2018 in China[J]. Hepatology, 2020, 71( 5): 1851- 1864. DOI: 10.1002/hep.31150. [25] YOUNOSSI ZM. Non-alcoholic fatty liver disease- A global public health perspective[J]. J Hepatol, 2019, 70( 3): 531- 544. DOI: 10.1016/j.jhep.2018.10.033. [26] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. [27] POUWELS S, SAKRAN N, GRAHAM Y, et al. Non-alcoholic fatty liver disease(NAFLD): A review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22( 1): 63. DOI: 10.1186/s12902-022-00980-1. [28] ZHU LH, WU X, LIAO RR. Mechanism and regulation of mitophagy in nonalcoholic fatty liver disease(NAFLD): A mini-review[J]. Life Sci, 2023, 312: 121162. DOI: 10.1016/j.lfs.2022.121162. [29] ZHOU T, CHANG L, LUO Y, et al. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy[J]. Redox Biol, 2019, 21: 101120. DOI: 10.1016/j.redox.2019.101120. [30] LI RB, XIN T, LI DD, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy[J]. Redox Biol, 2018, 18: 229- 243. DOI: 10.1016/j.redox.2018.07.011. [31] CAI JZ, HUANG JQ, YANG J, et al. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1-MFN2 pathway and Parkin mitophagy[J]. Cell Mol Life Sci, 2022, 79( 7): 354. DOI: 10.1007/s00018-022-04385-0. [32] LI XW, SHI Z, ZHU YW, et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice[J]. Br J Pharmacol, 2020, 177( 15): 3591- 3607. DOI: 10.1111/bph.15083. [33] SHAN SL, SHEN ZY, SONG FY. Autophagy and acetaminophen-induced hepatotoxicity[J]. Arch Toxicol, 2018, 92( 7): 2153- 2161. DOI: 10.1007/s00204-018-2237-5. [34] MCGILL MR, JAESCHKE H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis[J]. Pharm Res, 2013, 30( 9): 2174- 2187. DOI: 10.1007/s11095-013-1007-6. [35] RAMACHANDRAN A, JAESCHKE H. Acetaminophen toxicity: Novel insights into mechanisms and future perspectives[J]. Gene Expr, 2018, 18( 1): 19- 30. DOI: 10.3727/105221617X15084371374138. [36] KON K, KIM JS, JAESCHKE H, et al. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes[J]. Hepatology, 2004, 40( 5): 1170- 1179. DOI: 10.1002/hep.20437. [37] COVER C, MANSOURI A, KNIGHT TR, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity[J]. J Pharmacol Exp Ther, 2005, 315( 2): 879- 887. DOI: 10.1124/jpet.105.088898. [38] YAN MZ, JIN SW, LIU YG, et al. Cajaninstilbene acid ameliorates acetaminophen-induced liver injury through enhancing Sestrin2/AMPK-mediated mitochondrial quality control[J]. Front Pharmacol, 2022, 13: 824138. DOI: 10.3389/fphar.2022.824138. [39] TAN YL, HO HK. Hypothermia advocates functional mitochondria and alleviates oxidative stress to combat acetaminophen-induced hepatotoxicity[J]. Cells, 2020, 9( 11): 2354. DOI: 10.3390/cells9112354. [40] HAN S, YANG ZH, ZHANG T, et al. Epidemiology of alcohol-associated liver disease[J]. Clin Liver Dis, 2021, 25( 3): 483- 492. DOI: 10.1016/j.cld.2021.03.009. [41] ZHU L, LI HD, XU JJ, et al. Advancements in the alcohol-associated liver disease model[J]. Biomolecules, 2022, 12( 8): 1035. DOI: 10.3390/biom12081035. [42] WILLIAMS JA, DING WX. Role of autophagy in alcohol and drug-induced liver injury[J]. Food Chem Toxicol, 2020, 136: 111075. DOI: 10.1016/j.fct.2019.111075. [43] SILVA J, SPATZ MH, FOLK C, et al. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1α signaling axis[J]. Alcohol, 2021, 91: 1- 9. DOI: 10.1016/j.alcohol.2020.10.002. [44] ZHONG Z, LEMASTERS JJ. A unifying hypothesis linking hepatic adaptations for ethanol metabolism to the proinflammatory and profibrotic events of alcoholic liver disease[J]. Alcohol Clin Exp Res, 2018, 42( 11): 2072- 2089. DOI: 10.1111/acer.13877. [45] LU XY, XUAN WT, LI JJ, et al. AMPK protects against alcohol-induced liver injury through UQCRC2 to up-regulate mitophagy[J]. Autophagy, 2021, 17( 11): 3622- 3643. DOI: 10.1080/15548627.2021.1886829. [46] LI RB, DAI Z, LIU XM, et al. Interaction between dual specificity phosphatase-1 and cullin-1 attenuates alcohol-related liver disease by restoring p62-mediated mitophagy[J]. Int J Biol Sci, 2023, 19( 6): 1831- 1845. DOI: 10.7150/ijbs.81447. [47] NGUYEN MH, WONG G, GANE E, et al. Hepatitis B virus: Advances in prevention, diagnosis, and therapy[J]. Clin Microbiol Rev, 2020, 33( 2): e00046-19. DOI: 10.1128/CMR.00046-19. [48] LOUREIRO D, TOUT I, NARGUET S, et al. Mitochondrial stress in advanced fibrosis and cirrhosis associated with chronic hepatitis B, chronic hepatitis C, or nonalcoholic steatohepatitis[J]. Hepatology, 2023, 77( 4): 1348- 1365. DOI: 10.1002/hep.32731. [49] KIM SJ, KHAN M, QUAN J, et al. Hepatitis B virus disrupts mitochondrial dynamics: Induces fission and mitophagy to attenuate apoptosis[J]. PLoS Pathog, 2013, 9( 12): e1003722. DOI: 10.1371/journal.ppat.1003722. [50] LUCIFORA J, ARZBERGER S, DURANTEL D, et al. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection[J]. J Hepatol, 2011, 55( 5): 996- 1003. DOI: 10.1016/j.jhep.2011.02.015. [51] HUANG XY, LI D, CHEN ZX, et al. Hepatitis B Virus X protein elevates Parkin-mediated mitophagy through Lon Peptidase in starvation[J]. Exp Cell Res, 2018, 368( 1): 75- 83. DOI: 10.1016/j.yexcr.2018.04.016. [52] YOO YS, PARK YJ, LEE HS, et al. Mitochondria ubiquitin ligase, MARCH5 resolves hepatitis B virus X protein aggregates in the liver pathogenesis[J]. Cell Death Dis, 2019, 10( 12): 938. DOI: 10.1038/s41419-019-2175-z. [53] NING XJ, YAN X, WANG YF, et al. Parkin deficiency elevates hepatic ischemia/reperfusion injury accompanying decreased mitochondrial autophagy, increased apoptosis, impaired DNA damage repair and altered cell cycle distribution[J]. Mol Med Rep, 2018, 18( 6): 5663- 5668. DOI: 10.3892/mmr.2018.9606. [54] LIU Z, WANG MM, WANG X, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury[J]. Redox Biol, 2022, 52: 102305. DOI: 10.1016/j.redox.2022.102305. [55] LI HT, PAN YT, WU HJ, et al. Inhibition of excessive mitophagy by N-acetyl-L-tryptophan confers hepatoprotection against Ischemia-Reperfusion injury in rats[J]. PeerJ, 2020, 8: e8665. DOI: 10.7717/peerj.8665. [56] SAMANT H, AMIRI HS, ZIBARI GB. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management[J]. J Gastrointest Oncol, 2021, 12( Suppl 2): S361- S373. DOI: 10.21037/jgo.2020.02.08. [57] ANWANWAN D, SINGH SK, SINGH S, et al. Challenges in liver cancer and possible treatment approaches[J]. Biochim Biophys Acta Rev Cancer, 2020, 1873( 1): 188314. DOI: 10.1016/j.bbcan.2019.188314. [58] YANG WS, ZENG XF, LIU ZN, et al. Diet and liver cancer risk: A narrative review of epidemiological evidence[J]. Br J Nutr, 2020, 124( 3): 330- 340. DOI: 10.1017/S0007114520001208. [59] CHEN Y, CHEN HN, WANG K, et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma[J]. J Hepatol, 2019, 70( 1): 66- 77. DOI: 10.1016/j.jhep.2018.09.022. [60] SU Q, WANG JJ, LIU F, et al. Blocking Parkin/PINK1-mediated mitophagy sensitizes hepatocellular carcinoma cells to sanguinarine-induced mitochondrial apoptosis[J]. Toxicol In Vitro, 2020, 66: 104840. DOI: 10.1016/j.tiv.2020.104840. [61] ZHENG YH, HUANG C, LU L, et al. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib[J]. J Hematol Oncol, 2021, 14( 1): 16. DOI: 10.1186/s13045-020-01029-3. [62] WU H, WANG T, LIU YQ, et al. Mitophagy promotes sorafenib resistance through hypoxia-inducible ATAD3A dependent Axis[J]. J Exp Clin Cancer Res, 2020, 39( 1): 274. DOI: 10.1186/s13046-020-01768-8. [63] LIU LJ, LV Z, XUE X, et al. Canonical WNT signaling activated by WNT7B contributes to L-HBs-mediated sorafenib resistance in hepatocellular carcinoma by inhibiting mitophagy[J]. Cancers(Basel), 2022, 14( 23): 5781. DOI: 10.3390/cancers14235781. -



 PDF下载 ( 762 KB)
PDF下载 ( 762 KB)

 下载:
下载:


