去乙酰化酶Sirtuins家族与放射性肝病的关系
DOI: 10.12449/JCH240233
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:宗也凯负责论文设计,撰写论文;刘江凯参与论文指导。
-
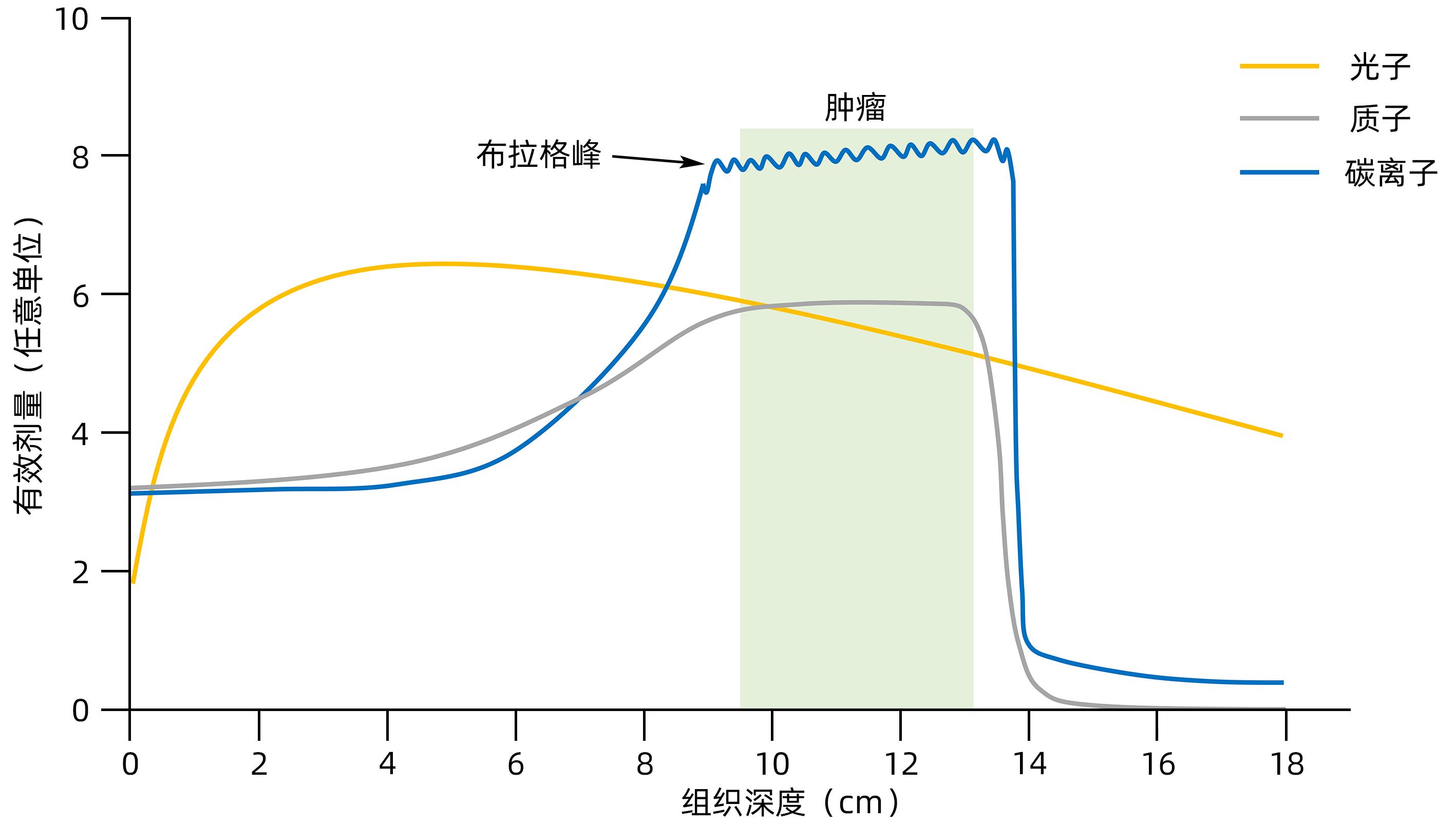
摘要: 放射性肝病(RILD)或称放射性肝炎,是一种由辐射引起的亚急性肝损伤。去乙酰化酶家族Sirtuins(SIRTs)作为衰老相关研究的焦点具有DNA修复和染色质调节等分子功能,是基因组和表观基因组稳定性的枢纽。辐射诱导的肝脏DNA损伤和反应是RILD主要的生理病理过程,这与SIRTs表征的功能相似。本文简述了SIRTs蛋白家族的结构和功能,回顾了放射治疗的物理生理学基本概念及进展,主要从放射生物学角度分析了SIRTs与RILD二者的内在关系,指出SIRTs作为RILD防治靶点的可能性。Abstract: Radiation-induced liver disease (RILD), also known as radiation hepatitis, is subacute liver injury induced by radiation. As the focus of senescence-related studies, the deacetylase family Sirtuins (SIRTs) have the molecular functions including DNA repair and chromatin regulation, which makes SIRTs a hub for regulating genome and epigenome stability. Radiation-induced hepatic DNA damage and reaction is the primary physiological and pathological process of RILD, which is similar to the function of SIRTs. This article briefly introduces the structure and function of the SIRTs protein family, elaborates on the basic concepts and progress of the physical physiology of radiation therapy, discusses the internal relationship between SIRTs and RILD from the perspective of radiobiology, and points out the possibility of SIRTs as a target for the prevention and treatment of RILD.
-
Key words:
- Radiation-Induced Liver Disease /
- Sirtuins /
- Radiation, Ionizing
-
[1] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [2] TOH MR, WONG EYT, WONG SH, et al. Global epidemiology and genetics of hepatocellular carcinoma[J]. Gastroenterology, 2023, 164( 5): 766- 782. DOI: 10.1053/j.gastro.2023.01.033. [3] LIEVENS Y, DEFOURNY N, CORRAL J, et al. How public health services pay for radiotherapy in Europe: An ESTRO-HERO analysis of reimbursement[J]. Lancet Oncol, 2020, 21( 1): e42- e54. DOI: 10.1016/S1470-2045(19)30794-6. [4] BASKAR R, ITAHANA K. Radiation therapy and cancer control in developing countries: Can we save more lives?[J]. Int J Med Sci, 2017, 14( 1): 13- 17. DOI: 10.7150/ijms.17288. [5] KOAY EJ, OWEN D, DAS P. Radiation-induced liver disease and modern radiotherapy[J]. Semin Radiat Oncol, 2018, 28( 4): 321- 331. DOI: 10.1016/j.semradonc.2018.06.007. [6] ZHOU YJ, TANG Y, LIU SJ, et al. Radiation-induced liver disease: Beyond DNA damage[J]. Cell Cycle, 2023, 22( 5): 506- 526. DOI: 10.1080/15384101.2022.2131163. [7] NAGARAJU GP, DARIYA B, KASA P, et al. Epigenetics in hepatocellular carcinoma[J]. Semin Cancer Biol, 2022, 86( Pt 3): 622- 632. DOI: 10.1016/j.semcancer.2021.07.017. [8] CANTÓ C, MENZIES KJ, AUWERX J. NAD(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus[J]. Cell Metab, 2015, 22( 1): 31- 53. DOI: 10.1016/j.cmet.2015.05.023. [9] AVALOS JL, BOEKE JD, WOLBERGER C. Structural basis for the mechanism and regulation of Sir2 enzymes[J]. Mol Cell, 2004, 13( 5): 639- 648. DOI: 10.1016/s1097-2765(04)00082-6. [10] WANG M, LIN HN. Understanding the function of mammalian sirtuins and protein lysine acylation[J]. Annu Rev Biochem, 2021, 90: 245- 285. DOI: 10.1146/annurev-biochem-082520-125411. [11] MORENO-YRUELA C, BÆK M, MONDA F, et al. Chiral posttranslational modification to lysine ε-amino groups[J]. Acc Chem Res, 2022, 55( 10): 1456- 1466. DOI: 10.1021/acs.accounts.2c00115. [12] BHEDA P, JING H, WOLBERGER C, et al. The substrate specificity of sirtuins[J]. Annu Rev Biochem, 2016, 85: 405- 429. DOI: 10.1146/annurev-biochem-060815-014537. [13] PAN CC, KAVANAGH BD, DAWSON LA, et al. Radiation-associated liver injury[J]. Int J Radiat Oncol Biol Phys, 2010, 76( 3 Suppl): S94- S100. DOI: 10.1016/j.ijrobp.2009.06.092. [14] MUNOZ-SCHUFFENEGGER P, NG S, DAWSON LA. Radiation-induced liver toxicity[J]. Semin Radiat Oncol, 2017, 27( 4): 350- 357. DOI: 10.1016/j.semradonc.2017.04.002. [15] GUHA CD, KAVANAGH BD. Hepatic radiation toxicity: Avoidance and amelioration[J]. Semin Radiat Oncol, 2011, 21( 4): 256- 263. DOI: 10.1016/j.semradonc.2011.05.003. [16] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [17] KIM J, JUNG Y. Radiation-induced liver disease: Current understanding and future perspectives[J]. Exp Mol Med, 2017, 49( 7): e359. DOI: 10.1038/emm.2017.85. [18] WEIGEL C, SCHMEZER P, PLASS C, et al. Epigenetics in radiation-induced fibrosis[J]. Oncogene, 2015, 34( 17): 2145- 2155. DOI: 10.1038/onc.2014.145. [19] DE LA PINTA ALONSO C. Radiation-induced liver disease in the era of SBRT: A review[J]. Expert Rev Gastroenterol Hepatol, 2020, 14( 12): 1195- 1201. DOI: 10.1080/17474124.2020.1814744. [20] DURANTE M, ORECCHIA R, LOEFFLER JS. Charged-particle therapy in cancer: Clinical uses and future perspectives[J]. Nat Rev Clin Oncol, 2017, 14( 8): 483- 495. DOI: 10.1038/nrclinonc.2017.30. [21] DURANTE M, FLANZ J. Charged particle beams to cure cancer: Strengths and challenges[J]. Semin Oncol, 2019, 46( 3): 219- 225. DOI: 10.1053/j.seminoncol.2019.07.007. [22] FOKAS E, KRAFT G, AN HX, et al. Ion beam radiobiology and cancer: Time to update ourselves[J]. Biochim Biophys Acta, 2009, 1796( 2): 216- 229. DOI: 10.1016/j.bbcan.2009.07.005. [23] WEI JL, WANG B, WANG HH, et al. Radiation-induced normal tissue damage: Oxidative stress and epigenetic mechanisms[J]. Oxid Med Cell Longev, 2019, 2019: 3010342. DOI: 10.1155/2019/3010342. [24] LOMAX ME, FOLKES LK, O’NEILL P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy[J]. Clin Oncol, 2013, 25( 10): 578- 585. DOI: 10.1016/j.clon.2013.06.007. [25] MANGONI M, BORGHESI S, ARISTEI C, et al. Radiobiology of stereotactic radiotherapy[J]. Rep Pract Oncol Radiother, 2022, 27( 1): 57- 62. DOI: 10.5603/rpor.a2022.0005. [26] WEI YL, WANG Y, JIA YM, et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes[J]. Science, 2021, 371( 6532): eabb1625. DOI: 10.1126/science.abb1625. [27] LI TM, CAO YL, LI B, et al. The biological effects of radiation-induced liver damage and its natural protective medicine[J]. Prog Biophys Mol Biol, 2021, 167: 87- 95. DOI: 10.1016/j.pbiomolbio.2021.06.012. [28] HUANG RX, ZHOU PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer[J]. Signal Transduct Target Ther, 2020, 5( 1): 60. DOI: 10.1038/s41392-020-0150-x. [29] GONG LY, ZHANG YJ, LIU CC, et al. Application of radiosensitizers in cancer radiotherapy[J]. Int J Nanomedicine, 2021, 16: 1083- 1102. DOI: 10.2147/IJN.S290438. [30] XIE YX, ZHANG JH, YE S, et al. SirT1 regulates radiosensitivity of hepatoma cells differently under normoxic and hypoxic conditions[J]. Cancer Sci, 2012, 103( 7): 1238- 1244. DOI: 10.1111/j.1349-7006.2012.02285.x. [31] LAEMMLE A, LECHLEITER A, ROH V, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions[J]. PLoS One, 2012, 7( 3): e33433. DOI: 10.1371/journal.pone.0033433. [32] CHEN XJ, HUAN HB, LIU CG, et al. Deacetylation of β-catenin by SIRT1 regulates self-renewal and oncogenesis of liver cancer stem cells[J]. Cancer Lett, 2019, 463: 1- 10. DOI: 10.1016/j.canlet.2019.07.021. [33] CUNEO KC, MORGAN MA, DAVIS MA, et al. Wee1 kinase inhibitor AZD1775 radiosensitizes hepatocellular carcinoma regardless of TP53 mutational status through induction of replication stress[J]. Int J Radiat Oncol Biol Phys, 2016, 95( 2): 782- 790. DOI: 10.1016/j.ijrobp.2016.01.028. [34] de MATTEIS S, SCARPI E, GRANATO AM, et al. Role of SIRT-3, p-mTOR and HIF-1α in hepatocellular carcinoma patients affected by metabolic dysfunctions and in chronic treatment with metformin[J]. Int J Mol Sci, 2019, 20( 6): 1503. DOI: 10.3390/ijms20061503. [35] FANG Y, ZHAN YZ, XIE YW, et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC[J]. Hepatology, 2022, 75( 6): 1386- 1401. DOI: 10.1002/hep.32177. [36] LIU Y, QI M, LIU LC, et al. Blocking Adipor1 enhances radiation sensitivity in hepatoma carcinoma cells[J]. Arch Biochem Biophys, 2022, 718: 109152. DOI: 10.1016/j.abb.2022.109152. [37] BAMODU OA, CHANG HL, ONG JR, et al. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma[J]. Cells, 2020, 9( 3): 746. DOI: 10.3390/cells9030746. [38] XU G, ZHU LH, WANG Y, et al. Stattic enhances radiosensitivity and reduces radio-induced migration and invasion in HCC cell lines through an apoptosis pathway[J]. Biomed Res Int, 2017, 2017: 1832494. DOI: 10.1155/2017/1832494. [39] TAO RD, COLEMAN MC, PENNINGTON JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress[J]. Mol Cell, 2010, 40( 6): 893- 904. DOI: 10.1016/j.molcel.2010.12.013. [40] REN T, ZHANG H, WANG J, et al. MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells[J]. Oncogene, 2017, 36( 42): 5897- 5909. DOI: 10.1038/onc.2017.167. [41] LIU Y, LIU YL, CHENG W, et al. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects[J]. Eur Rev Med Pharmacol Sci, 2017, 21( 5): 978- 998. [42] LIU XZ, REN SC, LI ZZ, et al. Sirt6 mediates antioxidative functions by increasing Nrf2 abundance[J]. Exp Cell Res, 2023, 422( 1): 113409. DOI: 10.1016/j.yexcr.2022.113409. [43] FURUKAWA A, TADA-OIKAWA S, KAWANISHI S, et al. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion[J]. Cell Physiol Biochem, 2007, 20( 1-4): 45- 54. DOI: 10.1159/000104152. [44] YE TJ, LU YL, YAN XF, et al. High mobility group box-1 release from H2O2-injured hepatocytes due to sirt1 functional inhibition[J]. World J Gastroenterol, 2019, 25( 36): 5434- 5450. DOI: 10.3748/wjg.v25.i36.5434. [45] LIU JX, LI D, ZHANG T, et al. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity[J]. Cell Death Dis, 2017, 8( 10): e3158. DOI: 10.1038/cddis.2017.564. [46] MELIN N, YARAHMADOV T, SANCHEZ-TALTAVULL D, et al. A new mouse model of radiation-induced liver disease reveals mitochondrial dysfunction as an underlying fibrotic stimulus[J]. JHEP Rep, 2022, 4( 7): 100508. DOI: 10.1016/j.jhepr.2022.100508. [47] REN JH, CHEN X, ZHOU L, et al. Protective role of Sirtuin3(SIRT3) in oxidative stress mediated by hepatitis B virus X protein expression[J]. PLoS One, 2016, 11( 3): e0150961. DOI: 10.1371/journal.pone.0150961. [48] HUANG RX, ZHOU PK. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy[J]. Signal Transduct Target Ther, 2021, 6( 1): 254. DOI: 10.1038/s41392-021-00648-7. [49] PALACIOS JA, HERRANZ D, DE BONIS ML, et al. SIRT1 contributes to telomere maintenance and augments global homologous recombination[J]. J Cell Biol, 2010, 191( 7): 1299- 1313. DOI: 10.1083/jcb.201005160. [50] GAO Y, TAN J, JIN JY, et al. SIRT6 facilitates directional telomere movement upon oxidative damage[J]. Sci Rep, 2018, 8( 1): 5407. DOI: 10.1038/s41598-018-23602-0. [51] JEONG SM, XIAO CY, FINLEY LWS, et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism[J]. Cancer Cell, 2013, 23( 4): 450- 463. DOI: 10.1016/j.ccr.2013.02.024. [52] MAO ZY, HINE C, TIAN X, et al. SIRT6 promotes DNA repair under stress by activating PARP1[J]. Science, 2011, 332( 6036): 1443- 1446. DOI: 10.1126/science.1202723. [53] VAN METER M, SIMON M, TOMBLINE G, et al. JNK phosphorylates SIRT6 to stimulate DNA double-strand break repair in response to oxidative stress by recruiting PARP1 to DNA breaks[J]. Cell Rep, 2016, 16( 10): 2641- 2650. DOI: 10.1016/j.celrep.2016.08.006. [54] VAZQUEZ BN, THACKRAY JK, SIMONET NG, et al. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair[J]. EMBO J, 2016, 35( 14): 1488- 1503. DOI: 10.15252/embj.201593499. [55] REZAZADEH S, YANG D, BIASHAD S ALI, et al. SIRT6 mono-ADP ribosylates KDM2A to locally increase H3K36me2 at DNA damage sites to inhibit transcription and promote repair[J]. Aging, 2020, 12( 12): 11165- 11184. DOI: 10.18632/aging.103567. [56] CHEN Y, ZHANG HP, XU Z, et al. A PARP1-BRG1-SIRT1 axis promotes HR repair by reducing nucleosome density at DNA damage sites[J]. Nucleic Acids Res, 2019, 47( 16): 8563- 8580. DOI: 10.1093/nar/gkz592. [57] YAMAGATA K, KITABAYASHI I. Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX[J]. Biochem Biophys Res Commun, 2009, 390( 4): 1355- 1360. DOI: 10.1016/j.bbrc.2009.10.156. [58] LEE N, RYU HG, KWON JH, et al. SIRT6 depletion suppresses tumor growth by promoting cellular senescence induced by DNA damage in HCC[J]. PLoS One, 2016, 11( 11): e0165835. DOI: 10.1371/journal.pone.0165835. [59] SERRANO L, MARTÍNEZ-REDONDO P, MARAZUELA-DUQUE A, et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation[J]. Genes Dev, 2013, 27( 6): 639- 653. DOI: 10.1101/gad.211342.112. [60] ZHANG WY, FENG YL, GUO QQ, et al. SIRT1 modulates cell cycle progression by regulating CHK2 acetylation-phosphorylation[J]. Cell Death Differ, 2020, 27( 2): 482- 496. DOI: 10.1038/s41418-019-0369-7. [61] LIU TZ, LIN YH, LENG WC, et al. A divergent role of the SIRT1-TopBP1 axis in regulating metabolic checkpoint and DNA damage checkpoint[J]. Mol Cell, 2014, 56( 5): 681- 695. DOI: 10.1016/j.molcel.2014.10.007. -



 PDF下载 ( 693 KB)
PDF下载 ( 693 KB)

 下载:
下载:


