熊去氧胆酸对慢性乙型肝炎患者新型冠状病毒感染的预防和治疗效果分析
DOI: 10.12449/JCH240309
Efficacy of ursodeoxycholic acid in the prevention and treatment of COVID-19 in patients with chronic hepatitis B
-
摘要:
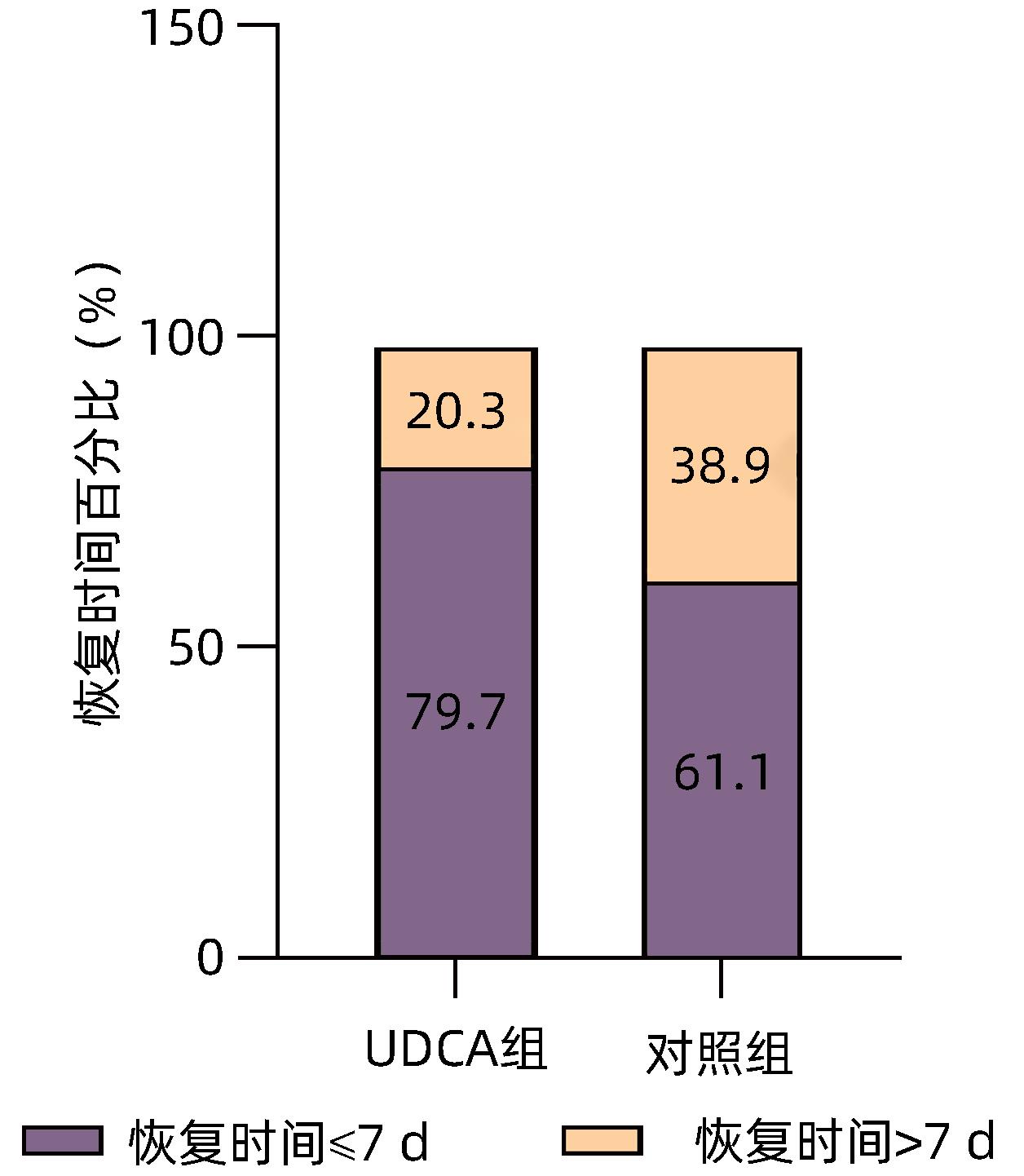
目的 探讨慢性乙型肝炎患者服用熊去氧胆酸后对新型冠状病毒感染(COVID-19)的潜在防治效果。 方法 收集2022年1月—12月首都医科大学附属北京地坛医院324例慢性乙型肝炎患者的临床资料,根据是否服用熊去氧胆酸,分为熊去氧胆酸组和对照组。利用倾向性评分匹配法(PSM)平衡两组患者年龄、性别和慢性并发症等混杂因素,观察两组间SARS CoV-2感染率、COVID-19后症状和恢复时间的差异。对白细胞、血红蛋白、血小板、ALT、AST、Alb、ALP、TBil、甘油三酯(TG)、总胆固醇(TC)等实验室指标、疫苗接种情况和COVID-19后肝病症状的发生情况进行对比。计量资料符合正态性分布的两组间比较采用成组t检验;偏态分布两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验和连续校正χ2检验。采用二元Logistic回归进行单因素分析和多因素分析,分析匹配后影响COVID-19的因素。 结果 熊去氧胆酸组87例患者,对照组237例患者,PSM后熊去氧胆酸组为78例,对照组为137例,两组间平衡性良好。熊去氧胆酸组SARS CoV-2感染率为82.1%(64/78),对照组感染率为95.6%(131/137),差异有统计学意义(χ2=10.847,P=0.001)。COVID-19后熊去氧胆酸组发生寒战(10.9% vs 38.9%,χ2=16.124,P<0.001)、咳嗽(56.3% vs 74.8%,χ2=6.889,P=0.009)的患者比例均小于对照组,差异具有统计学意义。COVID-19后熊去氧胆酸组患者恢复时间≤7天者比例达79.7%,对照组为61.1%,两组间差异具有统计学意义(χ2=6.760,P=0.009)。单因素和多因素Logistic回归分析均显示熊去氧胆酸是COVID-19的独立影响因素(OR值分别为0.21、0.17,P值均<0.05)。 结论 在慢性乙型肝炎患者中,服用熊去氧胆酸是COVID-19的保护因素,可一定程度上减轻相关症状,缩短恢复时间,在防治COVID-19方面具有重要价值。 Abstract:Objective To investigate the potential effect of ursodeoxycholic acid (UDCA) in the prevention and treatment of COVID-19 in patients with chronic hepatitis B. Methods Clinical data were collected from 324 patients with chronic hepatitis B who were treated in Beijing Ditan Hospital, Capital Medical University, from January to December 2022, and according to whether UDCA was administered, they were divided into UDCA group and control group. The propensity score matching (PSM) method was used to balance the confounding factors such as age, sex, and chronic complications, and the two groups were compared in terms of SARS-CoV-2 infection rate, symptoms, and recovery time after COVID-19. The two groups were also compared in terms of related laboratory markers (white blood cell count [WBC], hemoglobin [Hb], platelet count [PLT], alanine aminotransferase [ALT], aspartate aminotransferase [AST], albumin [Alb], alkaline phosphatase [ALP], total bilirubin [TBil], triglyceride [TG], and total cholesterol [TC]), vaccination, and the incidence rate of liver disease symptoms after COVID-19. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of data with skewed distribution between the two groups; the chi-square test and the continuously corrected chi-square test were used for comparison of categorical data between two groups. The binary Logistic regression model was used for univariate and multivariate analyses to investigate the influencing factors for COVID-19 after matching. Results There were 87 patients in the UDCA group and 237 patients in the control group, and after PSM, there were 78 patients in the UDCA group and 137 patients in the control group, with good balance between the two groups. There was a significant difference in SARS-CoV-2 infection rate between the UDCA group and the control group [82.1% (64/78) vs 95.6% (131/137), χ2=10.847, P=0.001]. After COVID-19, compared with the control group, the UDCA group had a significantly lower proportion of the patients with chill (10.9% vs 38.9%, χ2=16.124, P<0.001) and cough (56.3% vs 74.8%, χ2=6.889, P=0.009). There was a significant difference between the UDCA group and the control group in the proportion of the patients with a recovery time of ≤7 days after COVID-19 (79.7% vs 61.1%, χ2=6.760, P=0.009). Both univariate and multivariate logistic regression analyses showed that UDCA was an independent influencing factor for COVID-19 (odds ratio=0.21 and 0.17, both P<0.05). Conclusion UDCA is an protective factor against COVID-19 in patients with chronic hepatitis B and can alleviate related symptoms to some extent and shorten the recovery time, and therefore, it has an important value in the prevention and treatment of COVID-19. -
Key words:
- Hepatitis B, Chronic /
- SARS-CoV-2 /
- Ursodeoxycholic Acid /
- Propensity Score
-
表 1 UDCA组和对照组匹配前后基线特征
Table 1. Baseline characteristics before and after matching between UDCA group and control group
变量 匹配前 匹配后 UDCA组(n=87) 对照组(n=237) P值 UDCA组(n=78) 对照组(n=137) P值 年龄(岁) 54.6±11.4 50.3±11.3 0.003 54.0±11.7 54.0±11.5 0.895 BMI(kg/m2) 23.8±3.3 24.1±3.2 0.565 24.0±3.5 24.0±3.4 0.897 男性[例(%)] 44(50.6) 124(52.3) 0.780 42(53.8) 72(52.6) 0.855 吸烟[例(%)] 30(34.5) 84(35.4) 0.873 28(35.9) 48(35.0) 0.899 饮酒[例(%)] 27(31.0) 75(31.6) 0.916 25(32.1) 44(32.1) 0.992 慢性并发症[例(%)] 高血压 25(28.7) 55(23.2) 0.306 21(26.9) 38(27.7) 0.898 糖尿病 17(19.5) 42(17.7) 0.707 17(21.8) 28(20.4) 0.814 心血管疾病 10(11.5) 18(7.6) 0.268 9(11.5) 16(11.7) 0.975 肝硬化 39(44.8) 48(20.3) <0.001 30(38.5) 43(31.4) 0.292 表 2 匹配后UDCA组和对照组实验室指标和疫苗接种情况
Table 2. Laboratory indicators and vaccination status of UDCA administration group and control group after matching
项目 UDCA组(n=78) 对照组(n=137) 统计值 P值 实验室指标 ALT(U/L) 20.0(15.8~33.0) 23.0(16.5~36.0) Z=-1.180 0.238 AST(U/L) 29.0(21.0~41.2) 25.0(19.0~38.0) Z=-1.532 0.126 Alb(g/L) 40.5(33.8~45.0) 44.0(37.5~47.0) Z=-2.820 0.005 ALP(U/L) 100.5(80.8~130.5) 75.0(58.0~108.0) Z=-4.848 <0.001 TBil(μmol/L) 19.0(12.0~42.2) 16.0(12.0~27.5) Z=-1.796 0.072 TC(mmol/L) 4.0±1.3 4.2±1.2 t=0.702 0.484 TG(mmol/L) 1.1(0.8~1.6) 1.1(0.7~1.6) Z=-0.472 0.637 WBC(×109/L) 4.9(3.6~6.3) 4.9(3.8~6.4) Z=-0.310 0.756 Hb(g/L) 132.0(113.0~150.3) 135.0(116.5~148.0) Z=-0.445 0.567 PLT(×109/L) 142.0(69.8~228.8) 158.0(105.0~213.5) Z=-1.215 0.224 疫苗接种情况[例(%)] Z=-3.158 0.002 0针 49(62.8) 56(40.9) 1针 0(0.0) 5(3.6) 2针 8(10.3) 11(8.0) 3针 21(26.9) 64(46.7) 4针 0(0.0) 1(0.7) 表 3 UDCA组与对照组COVID-19情况
Table 3. COVID-19 infection in UDCA group and control group
项目 UDCA组(n=78) 对照组(n=137) χ2值 P值 COVID-19[例(%)] 64(82.1) 131(95.6) 10.847 0.001 COVID-19症状 发热[例(%)] 62(96.9) 126(96.2) 0.059 0.807 最高温度(℃) 38.6(38.1~39.0) 38.8(38.5~39.0) -3.081 0.078 发热时长(d) 2.0(1.0~2.0) 2.0(1.5~3.0) -3.364 0.039 寒战[例(%)] 7(10.9) 51(38.9) 16.124 <0.001 咳嗽[例(%)] 36(56.3) 98(74.8) 6.889 0.009 鼻塞[例(%)] 2(3.1) 10(7.6) 1.513 0.219 头痛[例(%)] 11(17.2) 10(7.6) 4.084 0.043 咽痛[例(%)] 31(48.4) 82(62.6) 3.537 0.060 肌肉痛[例(%)] 24(37.5) 58(44.3) 0.810 0.368 嗅觉障碍[例(%)] 19(29.7) 52(39.7) 1.860 0.173 疲乏[例(%)] 36(56.3) 64(48.9) 0.941 0.332 呼吸困难[例(%)] 1(1.6) 7(5.3) 1.562 0.211 恶心[例(%)] 3(4.7) 3(2.3) 0.829 0.363 腹泻[例(%)] 2(3.1) 1(0.8) 1.583 0.208 表 4 Logistic回归分析乙型肝炎患者COVID-19的影响因素
Table 4. Logistic regression analysis of hepatitis B patients infected with COVID-19
变量 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 UDCA 0.21(0.08~0.57) 0.002 0.17(0.06~0.49) 0.001 年龄(≥50岁) 0.77(0.28~2.10) 0.600 0.52(0.17~1.55) 0.239 BMI 0.96(0.85~1.10) 0.573 男性 0.58(0.22~1.51) 0.264 吸烟 1.02(0.39~2.67) 0.973 饮酒 0.87(0.33~2.28) 0.770 高血压 3.72(0.84~16.55) 0.085 4.17(0.90~19.26) 0.067 糖尿病 5.54(0.72~42.52) 0.100 心血管疾病 0.72(0.20~2.66) 0.623 肝硬化 0.75(0.29~1.93) 0.550 疫苗接种情况 全程接种 1.60(0.19~13.44) 0.663 1.62(0.18~14.68) 0.666 加强针 0.68(0.26~1.75) 0.421 0.42(0.14~1.21) 0.109 注:OR,优势比;疫苗接种情况,以部分接种为参照。 表 5 UDCA组和对照组中COVID-19患者的肝病症状比较
Table 5. Comparison of liver disease symptoms in patients with COVID-19 in UDCA group and control group
症状 UDCA组(n=64) 对照组(n=131) χ2值 P值 肝区痛[例(%)] 2(3.1) 7(5.3) 0.481 0.488 腹水[例(%)] 2(3.1) 4(3.1) 0.001 0.978 水肿[例(%)] 1(1.6) 4(3.1) 0.383 0.536 -
[1] LI Q, GUAN X, WU P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia[J]. N Engl J Med, 2020, 382( 13): 1199- 1207. DOI: 10.1056/NEJMoa2001316. [2] GARG S, KIM L, WHITAKER M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019- COVID-NET, 14 States, March 1-30, 2020[J]. MMWR Morb Mortal Wkly Rep, 2020, 69( 15): 458- 464. DOI: 10.15585/mmwr.mm6915e3. [3] VERITY R, OKELL LC, DORIGATTI I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis[J]. Lancet Infect Dis, 2020, 20( 6): 669- 677. DOI: 10.1016/S1473-3099(20)30243-7. [4] YANG X, YU Y, XU J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study[J]. Lancet Respir Med, 2020, 8( 5): 475- 481. DOI: 10.1016/S2213-2600(20)30079-5. [5] WHO. Weekly epidemiological update on COVID-19- 22 March 2023[EB/OL].[ 2023-03-22]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-22-march-2023. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-22-march-2023 [6] LI G, de CLERCQ E. Therapeutic options for the 2019 novel coronavirus(2019-nCoV)[J]. Nat Rev Drug Discov, 2020, 19( 3): 149- 150. DOI: 10.1038/d41573-020-00016-0. [7] CHEN YC, HUANG WX. Expert consensus on the application of azvudine in the treatment of SARS-CoV-2 infection[J]. China Pharm, 2023, 32( 3): 1- 6. DOI: 10.3969/j.issn.1006-4931.2023.03.001.陈勇川, 黄文祥. 阿兹夫定片在新型冠状病毒感染救治中应用的专家共识[J]. 中国药业, 2023, 32( 3): 1- 6. DOI: 10.3969/j.issn.1006-4931.2023.03.001. [8] BREVINI T, MAES M, WEBB GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2[J]. Nature, 2023, 615( 7950): 134- 142. DOI: 10.1038/s41586-022-05594-0. [9] YE H, DENG SW. Clinical evaluation of taurine ursodeoxycholic acid in the treatment of chronic hepatitis B with overlapping hepatitis E infection[J]. Chin Foreign Med Res, 2019, 17( 8): 47- 48. DOI: 10.14033/j.cnki.cfmr.2019.08.022.叶昊, 邓盛微. 牛磺酸熊去氧胆酸治疗慢性乙型肝炎重叠戊型肝炎感染的临床价值评价[J]. 中外医学研究, 2019, 17( 8): 47- 48. DOI: 10.14033/j.cnki.cfmr.2019.08.022. [10] van den BOSSCHE L, HINDRYCKX P, DEVISSCHER L, et al. Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice[J]. Appl Environ Microbiol, 2017, 83( 7): e02766-16. DOI: 10.1128/AEM.02766-16. [11] SUN XL, HU X, ZHANG YT. Clinical application of ursodeoxycholic acid[J]. Chin J Pharmacov, 2022, 19( 10): 1149- 1153. DOI: 10.19803/j.1672-8629.20210604.孙雪林, 胡欣, 张亚同. 熊去氧胆酸的临床应用进展[J]. 中国药物警戒, 2022, 19( 10): 1149- 1153. DOI: 10.19803/j.1672-8629.20210604. [12] LI YG, ZHU ZH. Efficacy of tenofovir combined with ursodeoxycholic acid in outpatient treatment of chronic hepatitis B[J]. Health Med Res Prac, 2022, 19( 4): 22- 26, 37. DOI: 10.11986/j.issn.1673-873X.2022.04.006.李玉刚, 朱增红. 替诺福韦联合熊去氧胆酸对慢性乙型肝炎的疗效观察[J]. 保健医学研究与实践, 2022, 19( 4): 22- 26, 37. DOI: 10.11986/j.issn.1673-873X.2022.04.006. [13] REIFFEL JA. Propensity score matching: The‘devil is in the details’ where more may be hidden than you know[J]. Am J Med, 2020, 133( 2): 178- 181. DOI: 10.1016/j.amjmed.2019.08.055. [14] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [15] National Health Commission of the People’s Republic of China. Diagnosis and Treatment of COVID-19(Trial Version 10)[J]. Chin J Rational Drug Use, 2023, 20( 1): 1- 11. DOI: 10.3969/j.issn.2096-3327.2023.01.001.中华人民共和国国家卫生健康委员会. 新型冠状病毒感染诊疗方案(试行第十版)[J]. 中国合理用药探索, 2023, 20( 1): 1- 11. DOI: 10.3969/j.issn.2096-3327.2023.01.001. [16] ZAMPINO R, MELE F, FLORIO LL, et al. Liver injury in remdesivir-treated COVID-19 patients[J]. Hepatol Int, 2020, 14( 5): 881- 883. DOI: 10.1007/s12072-020-10077-3. [17] WANG Q, GUO Y, IKETANI S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5[J]. Nature, 2022, 608( 7923): 603- 608. DOI: 10.1038/s41586-022-05053-w. [18] QIN SL, WANG YT, CAI XJ, et al. Research progress on anti- coronavirus drugs[J]. Chin J Comp Med, 2022, 32( 6): 105- 110. DOI: 10.3969/j.issn.1671-7856.2022.06.016.秦聖乐, 王玉涛, 蔡雪君, 等. 抗冠状病毒药物研究进展[J]. 中国比较医学杂志, 2022, 32( 6): 105- 110. DOI: 10.3969/j.issn.1671-7856.2022.06.016. [19] GAZIANO L, GIAMBARTOLOMEI C, PEREIRA AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19[J]. Nat Med, 2021, 27( 4): 668- 676. DOI: 10.1038/s41591-021-01310-z. [20] FUCHS CD, TRAUNER M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology[J]. Nat Rev Gastroenterol Hepatol, 2022, 19( 7): 432- 450. DOI: 10.1038/s41575-021-00566-7. [21] WANG CH, YAO XW, WANG R, et al. The latest research progress of novel coronavirus“Omicron sub-variant BA.5”[J]. J Hanan Med Coll, 2022, 28( 20): 1521- 1525. DOI: 10.13210/j.cnki.jhmu.20220909.002.王彩红, 姚晓文, 王蓉, 等. 新冠病毒“奥密克戎亚变体BA.5”的最新研究进展[J]. 海南医学院学报, 2022, 28( 20): 1521- 1525. DOI: 10.13210/j.cnki.jhmu.20220909.002. [22] LEVIN EG, LUSTIG Y, COHEN C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months[J]. N Engl J Med, 2021, 385( 24): e84. DOI: 10.1056/NEJMoa2114583. [23] BERGWERK M, GONEN T, LUSTIG Y, et al. Covid-19 breakthrough infections in vaccinated health care workers[J]. N Engl J Med, 2021, 385( 16): 1474- 1484. DOI: 10.1056/NEJMoa2109072. [24] WANG SJ. Treatment of chronic hepatitis B focuses on improving disease resistance[J]. Guangming J Chin Med, 2012, 27( 8): 1497- 1499. DOI: 10.3969/j.issn.1003-8914.2012.08.003.王素君. 慢性乙型肝炎治疗重在提高抗病力[J]. 光明中医, 2012, 27( 8): 1497- 1499. DOI: 10.3969/j.issn.1003-8914.2012.08.003. [25] CHEN C. Zhang Xuqing: Can patients with liver disease be vaccinated with COVID-19 vaccine?[J]. Liver Doctor, 2021, 3: 8- 9.陈词. 张绪清: 肝病患者能否接种新冠肺炎疫苗?[J]. 肝博士, 2021, 3: 8- 9. [26] National Health Commission of the People’s Republic of China. Notice on further optimizing and implementing Measures for the prevention and control of COVID-19[EB/OL].[ 2022-12-07]. http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml. http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml中华人民共和国国家卫生健康委员会. 关于进一步优化落实新冠肺炎疫情防控措施的通知[EB/OL].[ 2022-12-07]. http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml. http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml [27] BEUERS U, TRAUNER M, JANSEN P, et al. New paradigms in the treatment of hepatic cholestasis: From UDCA to FXR, PXR and beyond[J]. J Hepatol, 2015, 62( 1 Suppl): S25- S37. DOI: 10.1016/j.jhep.2015.02.023. [28] LIU Y, FENG Y, REN J, et al. The influencing factors of abnormal liver function in patients with COVID-19 and its dynamic changes in different drug treatments[J]. J Capit Med Univ, 2022, 43( 3): 433- 439. DOI: 10.3969/j.issn.1006-7795.2022.03.017.刘遥, 冯颖, 任婕, 等. 新型冠状病毒肺炎患者肝功能异常的影响因素及其在不同药物治疗中的动态变化[J]. 首都医科大学学报, 2022, 43( 3): 433- 439. DOI: 10.3969/j.issn.1006-7795.2022.03.017. [29] DAVIES MA, MORDEN E, ROSSEAU P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa[J]. Int J Infect Dis, 2023, 127: 63- 68. DOI: 10.1016/j.ijid.2022.11.024. [30] LEI SY, PENG H, LUO XH. Pathogenic mechanism of liver injury caused by coronavirus disease 2019 and protective strategies for patients with viral hepatitis cirrhosis[J]. J Clin Hepatol, 2020, 36( 7): 1619- 1622. DOI: 10.3969/j.issn.1001-5256.2020.07.037.雷偲艺, 彭虹, 罗新华. 新型冠状病毒肺炎引起肝损伤的致病机制及病毒性肝炎肝硬化患者的防护策略[J]. 临床肝胆病杂志, 2020, 36( 7): 1619- 1622. DOI: 10.3969/j.issn.1001-5256.2020.07.037. -



 PDF下载 ( 795 KB)
PDF下载 ( 795 KB)

 下载:
下载:


