冷冻消融协同仑伐替尼和程序性死亡受体1单抗治疗不可切除肝细胞癌的效果及安全性分析
DOI: 10.12449/JCH240317
Efficacy and safety of cryoablation combined with lenvatinib and anti-PD-1 monoclonal antibody in treatment of unresectable hepatocellular carcinoma
-
摘要:
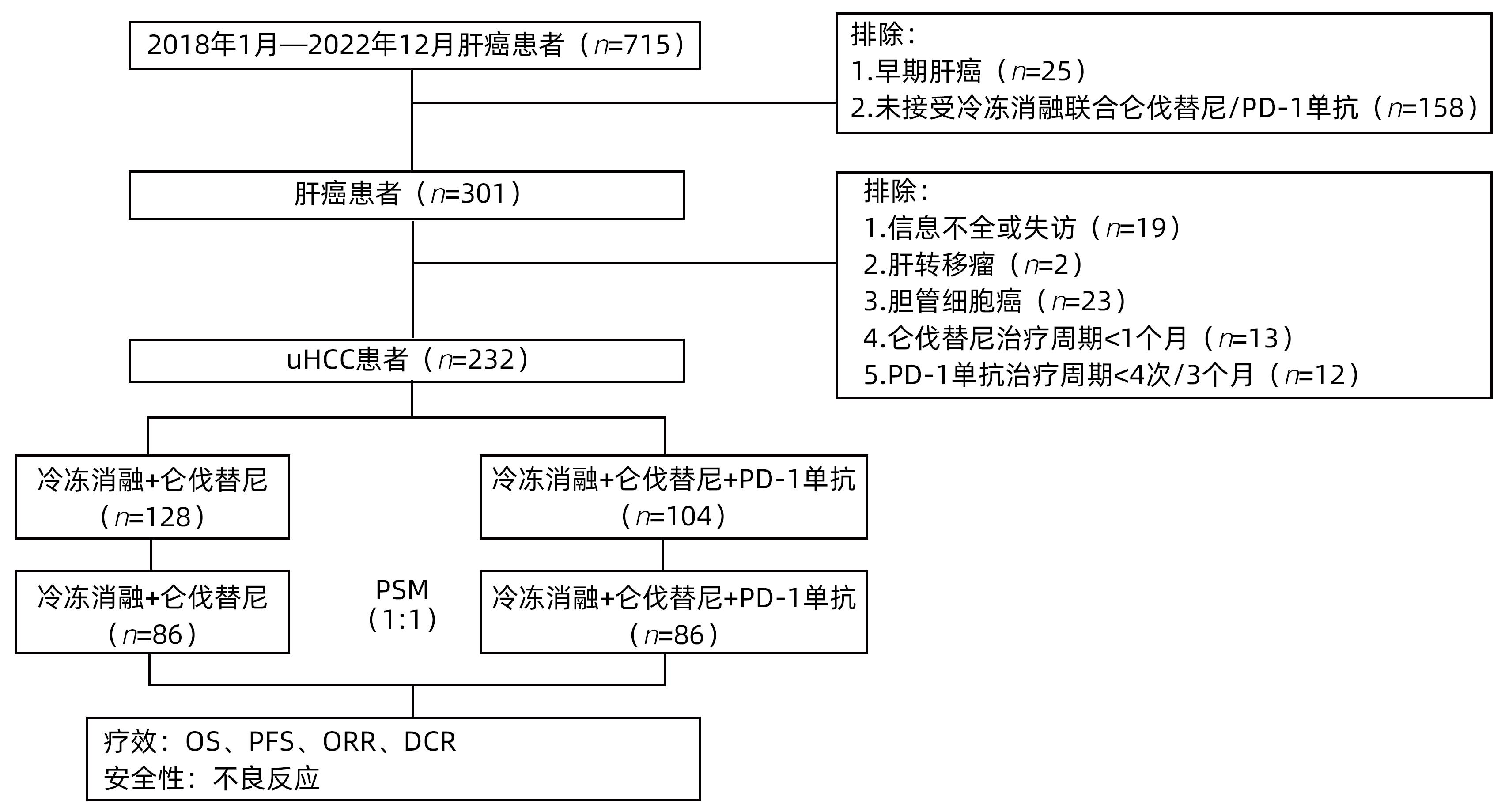
目的 分析程序性死亡受体1(PD-1)单抗是否提高冷冻消融联合仑伐替尼治疗不可切除性肝细胞癌(uHCC)患者的疗效和安全性。 方法 回顾性收集2018年1月—2022年12月在解放军总医院第五医学中心治疗的uHCC患者232例,其中128例接受冷冻消融联合仑伐替尼(二联)治疗,104例接受冷冻消融联合仑伐替尼和PD-1单抗(三联)治疗,用倾向性评分匹配方法(PSM)以1∶1进行匹配,经匹配后两组各86例。评估匹配后的2组患者客观缓解率(ORR)和疾病控制率(DCR)、总生存期(OS)、无进展生存期(PFS)和不良事件发生情况。定量资料若符合正态分布2组间比较采用成组t检验;非正态分布2组间比较采用Mann-Whitney U检验。定性资料采用χ2检验进行2组间比较。绘制生存曲线,运用Kaplan-Meier法计算2组患者的生存率,并利用Log-rank检验比较2组差异。通过Cox回归模型计算风险比(HR)和95%置信区间(95%CI),实现预后影响因素的单因素及多因素分析。 结果 中位随访时间为28个月,三联组死亡33例(38.0%),二联组死亡40例(46.0%)。三联治疗组的ORR和DCR较二联组明显增高(ORR:35.6% vs 14.5%,P=0.008;DCR:86.1% vs 64.1%,P=0.003)。三联组的OS和PFS较二联组均显著提高(P值分别为0.045、0.026)。单因素和多因素Cox风险比例模型分析显示治疗方案(HR=0.60,P=0.038)、AFP水平(HR=2.37,P=0.001)是影响OS的独立危险因素;治疗方案(HR=0.65,P=0.025)、糖尿病(HR=1.94,P=0.005)、之前是否接受过局部治疗(HR=0.63,P=0.014)、远处转移(HR=0.58,P=0.009)是影响PFS的独立危险因素。两组患者不良反应发生率相当,无明显差异(P值均>0.05)。 结论 对于uHCC患者,冷冻消融联合仑伐替尼和PD-1单抗三联治疗较冷冻消融联合仑伐替尼二联治疗显著提高了疗效,改善患者生存情况,而且不增加不良反应事件,为优化不可切除性肝癌的治疗方案提供了临床依据。 Abstract:Objective To investigate whether anti-PD-1 monoclonal antibody can improve the efficacy and safety of cryoablation combined with lenvatinib in the treatment of unresectable hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for 232 patients with unresectable HCC who were treated at The Fifth Medical Center of Chinese PLA General Hospital from January 2018 to December 2022, among whom 128 received cryoablation combined with lenvatinib (double combination) and 104 received cryoablation combined with lenvatinib and anti-PD-1 monoclonal antibody (triple combination). Propensity score matching was performed at a ratio of 1∶1, and finally there were 86 patients in each group. The two groups were evaluated in terms of objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), and adverse events (AEs). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Survival curves were plotted, and the Kaplan-Meier method was used to calculate the survival rate of patients in both groups, while the log-rank test was used for comparison between the two groups. The Cox regression model was used to calculate hazard ratio (HR) and 95% confidence interval (CI) and perform the univariate and multivariate analyses of influencing factors for prognosis. Results The median follow-up time was 28 months, and there were 33 deaths (38.0%) in the triple combination group and 40 deaths (46.0%) in the double combination group. Compared with the double combination group, the triple combination group had significantly higher ORR (35.6% vs 14.5%, P=0.008) and DCR (86.1% vs 64.1%, P=0.003). OS and PFS in the triple combination group were significantly higher than those in the double combination group (P=0.045 and 0.026). The univariate and multivariate Cox proportional-hazards regression model analyses showed that treatment regimen (HR=0.60, P=0.038) and alpha-fetoprotein level (HR=2.37, P=0.001) were independent risk factors for OS, and treatment regimen (HR=0.65, P=0.025), diabetes mellitus (HR=1.94, P=0.005), whether or not to have received local treatment (HR=0.63, P=0.014), and distant metastasis (HR=0.58, P=0.009) were independent risk factors for PFS. There was no significant difference in the incidence rate of AEs between the two groups (P>0.05). Conclusion For patients with unresectable HCC, the triple combination of cryoablation, lenvatinib, and anti-PD-1 monoclonal antibody significantly improves the treatment outcome and survival of patients compared with the double combination of cryoablation and lenvatinib, without increasing AEs, which provides a clinical basis for optimizing the treatment regimen for unresectable HCC. -
Key words:
- Carcinoma, Hepatocellular /
- Cryosurgery /
- Lenvatinib /
- Immune Checkpoint Inhibitors
-
表 1 冷冻消融+仑伐替尼+抗PD-1组(三联)和冷冻消融+仑伐替尼组(二联)PSM前后的临床特征
Table 1. Clinical features before and after PSM in Cryo+Lenvatinib+anti-PD-1 group and Cryo+Lenvatinib group
项目 PSM前 PSM后 合计(n=232) 三联 (n=104) 二联 (n=128) 统计值 P值 三联(n=86) 二联 (n=86) 统计值 P值 性别[例(%)] χ2=0.000 0.987 χ2=0.657 0.418 女 20(8.6) 9(8.7) 11(8.6) 9(10.5) 6(7.0) 男 212(91.4) 95(91.3) 117(91.4) 77(89.5) 80(93.0) 年龄[例(%)] χ2=0.100 0.752 χ2=0.102 0.749 <60岁 152(65.5) 67(64.4) 85(66.4) 55(64.0) 57(66.3) ≥60岁 80(34.5) 37(35.6) 43(33.6) 31(36.0) 29(33.7) BMI[例(%)] χ2=0.525 0.469 χ2=0.024 0.878 <24 kg/m2 102(44.0) 43(41.3) 59(46.1) 37(43.0) 38(44.2) ≥24 kg/m2 130(56.0) 61(58.7) 69(53.9) 49(57.0) 48(55.8) 门静脉高压[例(%)] χ2=0.009 0.926 χ2=0.000 >0.05 是 188(81.0) 84(80.8) 104(81.2) 70(81.4) 70(81.4) 否 44(19.0) 20(19.2) 24(18.8) 16(18.6) 16(18.6) 病因[例(%)] χ2=3.561 0.169 χ2=0.657 0.720 HBV 206(88.8) 96(92.3) 110(85.9) 80(93.0) 77(89.5) HCV 13(5.6) 2(1.9) 9(7.0) 2(2.3) 3(3.5) 其他 19(8.2) 6(5.8) 9(7.0) 4(4.7) 6(7.0) 抗病毒[例(%)] χ2=2.740 0.098 χ2=1.758 0.185 是 190(81.9) 90(86.5) 100(78.1) 72(83.7) 65(75.6) 否 42(18.1) 14(13.5) 28(21.9) 14(16.3) 21(24.4) AFP[例(%)] χ2=9.316 0.002 χ2=0.595 0.440 <400 ng/mL 126(54.3) 68(65.4) 58(45.3) 52(60.5) 47(54.7) ≥400 ng/mL 106(45.7) 36(34.6) 70(54.7) 34(39.5) 39(45.3) PLT(×109/L) 125.0 (90.0~187.0) 118.0(80.0~146.0) 142.0 (96.2~203.8) U=5 183 0.002 119.5 (88.8~155.8) 133.0 (92.8~203.3) U=3 180 0.115 ALT(U/L) 35.0 (22.0~57.0) 37.0 (24.0~74.5) 33.0 (19.5~53.1) U=5 826 0.121 36.0 (23.5~55.3) 33.5 (21.8~59.0) U=3 530 0.607 AST(U/L) 41.0 (28.0~77.9) 38.0 (27.3~78.8) 44.0 (28.3~77.5) U=6 583 0.747 37.0 (27.0~61.2) 37.0 (27.0~76.3) U=3 655 0.896 表 2 PSM后影响患者OS和PFS的Cox单因素及多因素回归分析
Table 2. Cox single and multi-factor regression analysis of influencing OS and PFS after PSM
项目 OS PFS 单因素分析 多因素分析 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 HR(95%CI) P值 HR(95%CI) P值 治疗方案(三联 vs 二联) 0.63(0.39~0.99) 0.047 0.60(0.37~0.97) 0.038 0.66(0.46~0.96) 0.028 0.65(0.44~0.95) 0.025 性别(男 vs 女) 0.73(0.31~1.68) 0.453 1.09(0.60~1.98) 0.782 年龄(≥60岁 vs <60岁) 0.88(0.54~1.43) 0.615 1.03(0.70~1.51) 0.886 BMI(≥24 kg/m2 vs <24 kg/m2) 1.49(0.93~2.39) 0.100 1.37(0.94~2.00) 0.099 Child-Pugh分级(B vs A) 0.95(0.58~1.57) 0.842 0.96(0.65~1.42) 0.847 BCLC分级(C vs B) 1.82(1.05~3.14) 0.032 0.86(0.33~2.23) 0.758 0.79(0.54~1.17) 0.234 糖尿病(有 vs 无) 0.70(0.37~1.30) 0.256 1.69(1.08~2.64) 0.022 1.94(1.22~3.09) 0.005 门静脉高压(有 vs 无) 2.08(1.03~4.20) 0.042 1.73(0.83~3.58) 0.142 0.89(0.57~1.41) 0.625 肝硬化(有 vs 无) 20.72(0.01~5 207.00) 0.448 0.81(0.20~3.27) 0.763 HBV(有 vs 无) 0.89(0.38~2.05) 0.776 0.88(0.45~1.75) 0.722 抗病毒(有 vs 无) 0.93(0.52~1.63) 0.789 0.94(0.59~1.48) 0.779 肿瘤数目(>1 vs 1) 0.96(0.54~1.73) 0.901 1.00(0.61~1.62) 0.988 肿瘤大小(≥7.07 cm vs <7.07 cm) 1.68(1.05~2.68) 0.030 1.18(0.70~1.98) 0.537 0.76(0.52~1.12) 0.161 AFP(≥400 ng/mL vs <400 ng/mL) 2.46(1.55~3.92) <0.001 2.37(1.44~3.88) 0.001 1.06(0.73~1.53) 0.772 之前局部治疗(有 vs 无) 0.55(0.34~0.87) 0.012 0.67(0.41~1.11) 0.123 0.63(0.44~0.91) 0.013 0.63(0.43~0.89) 0.014 之前系统治疗(有 vs 无) 0.64(0.26~1.58) 0.331 0.74(0.39~1.42) 0.372 后续局部治疗(有 vs 无) 0.41(0.10~1.70) 0.22 1.80(0.87~3.72) 0.110 后续系统治疗(有 vs 无) 0.39(0.16~0.97) 0.042 0.62(0.24~1.61) 0.325 1.17(0.72~1.89) 0.532 冷冻消融次数(>1 vs 1) 0.79(0.47~1.33) 0.382 1.56(0.99~2.45) 0.054 远处转移(有 vs 无) 1.16(0.72~1.86) 0.539 0.53(0.35~0.80) 0.002 0.58(0.38~0.87) 0.009 血管侵犯(有 vs 无) 1.86(1.11~3.12) 0.019 1.79(0.73~4.41) 0.204 0.89(0.61~1.30) 0.553 表 3 PSM前影响患者OS和PFS的Cox单及多因素回归分析
Table 3. Cox single and multi-factor regression analysis of influencing OS and PFS before PSM
项目 OS PFS 单因素分析 多因素分析 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 HR(95%CI) P值 HR(95%CI) P值 治疗方案(三联 vs 二联) 0.57(0.38~0.85) 0.005 0.63(0.41~0.98) 0.039 0.82(0.60~1.13) 0.228 0.77(0.54~1.10) 0.149 性别(男 vs 女) 0.86(0.42~1.78) 0.693 1.19(0.70~2.02) 0.526 年龄(≥60岁 vs <60岁) 0.83(0.55~1.26) 0.379 0.90(0.64~1.25) 0.520 BMI(≥24 kg/m2 vs <24 kg/m2) 1.31(0.88~1.96) 0.179 1.24(0.90~1.70) 0.190 Child-Pugh分级(B vs A) 1.22(0.81~1.84) 0.335 0.99 (0.71~1.38) 0.946 BCLC分级(C vs B) 1.69(1.07~2.65) 0.024 0.92(0.41~2.07) 0.846 0.79 (0.57~1.10) 0.171 0.61 (0.32~1.16) 0.132 糖尿病(有 vs 无) 0.72(0.43~1.22) 0.227 1.44(0.98~2.12) 0.062 门静脉高压(有 vs 无) 1.72(0.99~3.00) 0.053 0.89(0.60~1.31) 0.549 肝硬化(有 vs 无) 1.12(0.41~3.06) 0.819 1.04(0.46~2.35) 0.929 HBV(有 vs 无) 1.08(0.56~2.07) 0.826 0.97(0.58~1.60) 0.894 抗病毒(有 vs 无) 0.77(0.48~1.24) 0.285 0.86 (0.57~1.29) 0.464 肿瘤数目(>1 vs 1) 0.85(0.53~1.35) 0.487 1.09(0.73~1.62) 0.682 肿瘤大小(≥7.07 cm vs <7.07 cm) 1.83(1.24~2.71) 0.002 1.09(0.71~1.68) 0.689 0.76 (0.55~1.05) 0.100 0.71(0.49~1.02) 0.064 AFP(≥400 ng/mL vs<400 ng/mL) 2.11(1.42~3.12) <0.001 1.75(1.16~2.64) 0.008 0.94(0.69~1.29) 0.709 1.00(0.72~1.40) 0.989 之前局部治疗(有 vs 无) 0.45(0.30~0.68) <0.001 0.58(0.38~0.90) 0.014 0.84(0.61~1.15) 0.274 0.78 (0.56~1.09) 0.152 之前系统治疗(有 vs 无) 0.67(0.32~1.37) 0.270 0.91(0.55~1.50) 0.701 后续局部治疗(有 vs 无) 0.26(0.06~1.08) 0.064 1.63(0.88~3.01) 0.120 后续系统治疗(有 vs 无) 0.41(0.20~0.84) 0.015 0.66(0.30~1.42) 0.284 1.16(0.77~1.74) 0.489 1.18 (0.74~1.86) 0.491 冷冻消融次数(>1 vs 1) 0.62(0.41~0.95) 0.028 0.60(0.39~0.93) 0.023 1.43(0.97~2.10) 0.070 1.35 (0.91~2.00) 0.135 远处转移(有 vs 无) 0.91(0.60~1.38) 0.661 1.12(0.81~1.56) 0.498 血管侵犯(有 vs 无) 1.79(1.15~2.76) 0.009 1.73(0.78~3.82) 0.178 0.90(0.65~1.25) 0.523 1.42(0.74~2.71) 0.292 表 4 肿瘤反应
Table 4. Tumor response
肿瘤疗效评价 PSM前 PSM后 三联 二联 P值 三联 二联 P值 CR[例(%)] 9(8.7) 5(3.9) 0.131 7(8.1) 4(4.7) 0.350 PR[例(%)] 27(26.0) 13(10.2) 0.002 22(25.6) 10(11.6) 0.019 SD[例(%)] 50(48.1) 66(51.6) 0.597 43(50.0) 41(47.7) 0.760 PD[例(%)] 18(17.3) 44(34.4) 0.003 14(18.3) 31(36.0) 0.003 ORR[例(%)] 36(34.6) 14(14.1) 0.000 29(35.6) 14(14.5) 0.008 DCR[例(%)] 86(82.7) 84(65.6) 0.003 72(86.1) 55(64.1) 0.003 表 5 PSM后不良反应
Table 5. Adverse reactions after PSM
不良反应 1~5级 3~5级 三联(n=86) 二联(n=86) P值 三联(n=86) 二联(n=86) P值 高血压[例(%)] 22(25.6) 26(30.2) 0.497 5(5.8) 4(4.7) 0.899 手足综合征[例(%)] 19(22.1) 12(14.0) 0.165 3(3.5) 3(3.5) 0.676 乏力[例(%)] 18(20.9) 15(17.4) 0.561 2(2.3) 3(3.5) 0.375 肺炎[例(%)] 2(2.3) 0(0.0) 0.155 1(1.1) 0(0.0) 0.375 发热[例(%)] 7(8.1) 2(2.3) 0.087 1(1.1) 0(0.0) 0.375 腹胀[例(%)] 3(3.5) 3(3.5) >0.05 0(0.0) 0(0.0) 腹痛[例(%)] 7(8.1) 3(3.5) 0.192 1(1.1) 0(0.0) 0.375 腹泻[例(%)] 23(26.7) 21(24.4) 0.727 2(2.3) 2(2.3) 0.748 厌食[例(%)] 15(17.4) 18(20.9) 0.561 3(3.5) 2(2.3) 0.882 体质量下降[例(%)] 3(3.5) 1(1.2) 0.312 0(0.0) 0(0.0) 恶心呕吐[例(%)] 6(7.0) 7(8.1) 0.773 0(0.0) 0(0.0) 消化道出血[例(%)] 2(2.3) 0(0.0) 0.115 2(2.3) 0(0.0) 0.198 肾病综合征[例(%)] 2(2.3) 1(1.1) 0.560 1(1.1) 0(0.0) 0.375 脱皮[例(%)] 5(5.8) 3(3.5) 0.469 0(0.0) 1(1.1) 0.237 皮疹[例(%)] 10(11.6) 8(9.3) 0.618 1(1.1) 0(0.0) 0.375 肝区疼痛[例(%)] 7(8.1) 9(10.5) 0.600 0(0.0) 2(2.3) 0.086 肝性脑病[例(%)] 2(2.3) 1(1.2) 0.560 2(2.3) 1(1.1) 0.719 声音嘶哑[例(%)] 5(5.8) 2(2.3) 0.247 0(0.0) 0(0.0) 甲状腺功能减退[例(%)] 11(12.8) 8(9.3) 0.466 0(0.0) 1(1.1) 0.237 肝衰竭[例(%)] 0(0.0) 1(1.2) 0.316 0(0.0) 1(1.1) 0.237 蛋白尿[例(%)] 18(20.9) 13(15.1) 0.321 3(3.5) 4(4.7) 0.350 水肿[例(%)] 6(7.0) 6(7.0) >0.05 2(2.3) 3(3.5) 0.375 皮肤瘙痒[例(%)] 9(10.5) 7(8.1) 0.793 1(1.1) 1(1.1) 0.830 口腔溃疡[例(%)] 5(5.8) 2(2.3) 0.247 1(1.1) 1(1.1) 0.830 脱发[例(%)] 2(2.3) 0(0.0) 0.155 1(1.1) 0(0.0) 0.375 肌肉疼痛[例(%)] 2(2.3) 2(2.3) >0.05 0(0.0) 0(0.0) AST 升高[例(%)] 31(36.0) 21(24.4) 0.097 0(0.0) 0(0.0) ALT升高[例(%)] 29(33.7) 20(23.3) 0.128 5(5.8) 1(1.1) 0.125 高胆红素血症[例(%)] 25(29.1) 29(33.7) 0.511 2(2.3) 5(5.8) 0.061 低蛋白血症[例(%)] 35(40.7) 31(36.0) 0.531 5(5.8) 6(7.0) 0.256 腹水[例(%)] 1(1.2) 0(0.0) 0.316 1(0.0) 0(0.0) 0.375 白细胞减少症[例(%)] 21(24.4) 21(24.4) >0.05 5(5.8) 4(4.7) 0.899 PLT下降[例(%)] 4(4.7) 2(2.3) 0.406 1(1.1) 1(1.1) 0.830 -
[1] LLOVET JM, de BAERE T, KULIK L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 5): 293- 313. DOI: 10.1038/s41575-020-00395-0. [2] GHAVIMI S, APFEL T, AZIMI H, et al. Management and treatment of hepatocellular carcinoma with immunotherapy: a review of current and future options[J]. J Clin Transl Hepatol, 2020, 8( 2): 168- 176. DOI: 10.14218/JCTH.2020.00001. [3] LIU YE, ZONG J, CHEN XJ, et al. Cryoablation combined with radiotherapy for hepatic malignancy: Five case reports[J]. World J Gastrointest Oncol, 2020, 12( 2): 237- 247. DOI: 10.4251/wjgo.v12.i2.237. [4] KIM R, KANG TW, DI CHA, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications[J]. Eur Radiol, 2019, 29( 2): 654- 662. DOI: 10.1007/s00330-018-5617-6. [5] ABDO J, CORNELL DL, MITTAL SK, et al. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers[J]. Front Oncol, 2018, 8: 85. DOI: 10.3389/fonc.2018.00085. [6] CHEN LT, MARTINELLI E, CHENG AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO[J]. Ann Oncol, 2020, 31( 3): 334- 351. DOI: 10.1016/j.annonc.2019.12.001. [7] BENSON AB, D’ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 5): 541- 565. DOI: 10.6004/jnccn.2021.0022. [8] SCHREIBER RD, OLD LJ, SMYTH MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion[J]. Science, 2011, 331( 6024): 1565- 1570. DOI: 10.1126/science.1203486. [9] CHENG AL, QIN S, IKEDA M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma[J]. J Hepatol, 2022, 76( 4): 862- 873. DOI: 10.1016/j.jhep.2021.11.030. [10] PENG Z, FAN W, ZHU B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A Phase Ⅲ, Randomized Clinical Trial-LAUNCH)[J]. J Clin Oncol, 2023, 41( 1): 117- 127. DOI: 10.1200/JCO.22.00392. [11] KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation(TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69( 8): 1492- 1501. DOI: 10.1136/gutjnl-2019-318934. [12] DUFFY AG, ULAHANNAN SV, MAKOROVA-RUSHER O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma[J]. J Hepatol, 2017, 66( 3): 545- 551. DOI: 10.1016/j.jhep.2016.10.029. [13] NIU LZ, LI JL, ZENG JY, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer[J]. World J Gastroenterol, 2013, 19( 22): 3473- 3480. DOI: 10.3748/wjg.v19.i22.3473. [14] YAKKALA C, DENYS A, KANDALAFT L, et al. Cryoablation and immunotherapy of cancer[J]. Curr Opin Biotechnol, 2020, 65: 60- 64. DOI: 10.1016/j.copbio.2020.01.006. [15] TAN J, LIU T, FAN W, et al. Anti-PD-L1 antibody enhances curative effect of cryoablation via antibody-dependent cell-mediated cytotoxicity mediating PD-L1highCD11b+ cells elimination in hepatocellular carcinoma[J]. Acta Pharm Sin B, 2023, 13( 2): 632- 647. DOI: 10.1016/j.apsb.2022.08.006. [16] KWAK K, YU B, LEWANDOWSKI RJ, et al. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation[J]. Theranostics, 2022, 12( 5): 2175- 2204. DOI: 10.7150/thno.67530. [17] VOGEL A, MEYER T, SAPISOCHIN G, et al. Hepatocellular carcinoma[J]. Lancet, 2022, 400( 10360): 1345- 1362. DOI: 10.1016/S0140-6736(22)01200-4. [18] LU M, ZHANG X, GAO X, et al. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer[J]. Pharmacol Res, 2021, 174: 105829. DOI: 10.1016/j.phrs.2021.105829. [19] YANG XR, SUN HC, XIE Q, et al. Chinese expert guidance on overall application of lenvatinib in hepatocellular carcinoma[J]. Chin J Dig Surg, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035.杨欣荣, 孙惠川, 谢青, 等. 仑伐替尼肝癌全病程应用中国专家指导意见[J]. 中华消化外科杂志, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035. [20] YANG Y, LU Y, WANG C, et al. Cryotherapy is associated with improved clinical outcomes of Sorafenib therapy for advanced hepatocellular carcinoma[J]. Cell Biochem Biophys, 2012, 63( 2): 159- 169. DOI: 10.1007/s12013-012-9353-2. [21] WANG YY, YANG X, WANG YC, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma[J]. World J Gastroenterol, 2023, 29( 10): 1614- 1626. DOI: 10.3748/wjg.v29.i10.1614. [22] CAO F, YANG Y, SI T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study[J]. Front Oncol, 2021, 11: 783480. DOI: 10.3389/fonc.2021.783480. [23] CAI M, HUANG W, HUANG J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study[J]. Front Immunol, 2022, 13: 848387. DOI: 10.3389/fimmu.2022.848387. [24] PARK JW, KIM YJ, KIM DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase Ⅲ STAH trial[J]. J Hepatol, 2019, 70( 4): 684- 691. DOI: 10.1016/j.jhep.2018.11.029. [25] MARINELLI B, KIM E, D’ALESSIO A, et al. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study[J]. J Immunother Cancer, 2022, 10( 6). DOI: 10.1136/jitc-2021-004205. [26] YUAN Y, HE W, YANG Z, et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study[J]. Int J Surg, 2023, 109( 5): 1222- 1230. DOI: 10.1097/JS9.0000000000000256. [27] CUI W, FAN W, HUANG K, et al. Large hepatocellular carcinomas: treatment with transarterial chemoembolization alone or in combination with percutaneous cryoablation[J]. Int J Hyperthermia, 2018, 35( 1): 239- 245. DOI: 10.1080/02656736.2018.1493235. [28] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391( 10126): 1163- 1173. DOI: 10.1016/S0140-6736(18)30207-1. -



 PDF下载 ( 1848 KB)
PDF下载 ( 1848 KB)

 下载:
下载:







