抗甲状腺药物所致肝损伤的诊断与治疗原则
DOI: 10.12449/JCH240331
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:杨锐、杨睿涛负责课题设计,资料分析,撰写论文;邓勋、曾森祥、杨晓燕参与收集数据,修改论文;杨锐负责拟定写作思路,指导撰写文章并最后定稿。
-
摘要: 药物治疗是甲状腺功能亢进的主要治疗方法。抗甲状腺药物可引起肝损伤,药物性肝损伤的诊断多采用排他性诊断,诊断基于病史采集、临床症状、血清生化、影像学及组织学等。药物性肝损伤可依据肝损伤严重程度分为轻度、中度、重度和致命四种等级,轻者常不必停药,但需定期监测肝功能,重者可发生肝衰竭,病死率高,若能尽早发现、及时停药,辅以合理药物治疗,可避免致命后果。抗甲状腺药物性肝损伤的治疗原则是促进肝损伤恢复、防止肝损伤重症化、慢性化和降低死亡风险。规范用药、及时监测、早期发现和早期治疗是防治抗甲状腺药物肝损伤的重要举措。Abstract: Pharmacotherapy is the primary treatment method for hyperthyroidism. Antithyroid drugs can induce liver injury, and the diagnosis of drug-induced liver injury is mostly exclusive based on medical history collection, clinical symptoms, serum biochemistry, radiological examination, and histology. According to the severity of liver injury, drug-induced liver injury can be classified into mild, moderate, severe, and fatal degrees. Drug withdrawal may not be necessary for patients with mild liver injury, but regular monitoring of liver function is required; in severe cases, patients may develop liver failure, which may lead to a mortality rate, and early identification, timely drug withdrawal, and reasonable pharmacotherapy can help to avoid fatal consequences. The treatment principles of liver injury induced by antithyroid drugs include promoting the recovery of liver injury, preventing the severe exacerbation and chronicity of liver injury, and reducing the risk of death. Standardized medication, timely monitoring, early identification, and early treatment are important measures for the prevention and treatment of liver injury induced by antithyroid drugs.
-
Key words:
- Drug Induced Liver Injury /
- Methimazole /
- Propythiouracil /
- Hyperthyroidism
-
[1] SUZUKI N, NOH JY, HIRUMA M, et al. Analysis of antithyroid drug-induced severe liver injury in 18,558 newly diagnosed patients with Graves’ disease in Japan[J]. Thyroid, 2019, 29( 10): 1390- 1398. DOI: 10.1089/thy.2019.0045. [2] ZENG J, LUO F, LIN Z, et al. Rash and cholestatic liver injury caused by methimazole in a woman with Turner syndrome and Graves’s disease: a case report and literature review[J]. BMC Endocr Disord, 2021, 21( 1): 179. DOI: 10.1186/s12902-021-00819-1. [3] Technology Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association; Study Group of Drug⁃Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases. Guidelines for the diagnosis and treatment of drug-induced liver injury[J].[J]. J Clin Hepatol, 2015, 31( 11): 1752- 1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南[J]. 临床肝胆病杂志, 2015, 31( 11): 1752- 1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. [4] Professional Committee of Prevention and Treatment of Pharmaceutical Liver Injury of China Medical Biotechnology Association, Pharmaceutical Hepatology Group of Liver Disease Branch of Chinese Medical Association. Chinese guideline for diagnosis and management of drug-induced liver injury(2023 version)[J]. Chin J Gastroenterol, 2022, 27( 6): 341- 375. DOI: 10.3760/cma.j.cn501113-20230419-00176.中国医药生物技术协会药物性肝损伤防治技术专业委员会, 中华医学会肝病学分会药物性肝病学组. 中国药物性肝损伤诊治指南(2023年版)[J]. 胃肠病学, 2022, 27( 6): 341- 375. DOI: 10.3760/cma.j.cn501113-20230419-00176. [5] YU W, WU N, LI L, et al. Side effects of PTU and MMI in the treatment of hyperthyroidism: a systematic review and meta-analysis[J]. Endocr Pract, 2020, 26( 2): 207- 217. DOI: 10.4158/EP-2019-0221. [6] CARRION AF, CZUL F, AROSEMENA LR, et al. Propylthiouracil-induced acute liver failure: role of liver transplantation[J]. Int J Endocrinol, 2010, 2010: 910636. DOI: 10.1155/2010/910636. [7] MIKHAIL NE. Methimazole-induced cholestatic jaundice[J]. South Med J, 2004, 97( 2): 178- 182. DOI: 10.1111/j.1572-0241.2001.03469.x. [8] COOPER DS. Antithyroid drugs[J]. N Engl J Med, 2005, 352( 9): 905- 917. DOI: 10.1056/NEJMra042972. [9] HEIDARI R, NIKNAHAD H, JAMSHIDZADEH A, et al. Factors affecting drug-induced liver injury: antithyroid drugs as instances[J]. Clin Mol Hepatol, 2014, 20( 3): 237- 248. DOI: 10.1155/2010/910636. [10] MIZUTANI T, YOSHIDA K, MURAKAMI M, et al. Evidence for the involvement of N-methylthiourea, a ring cleavage metabolite, in the hepatotoxicity of methimazole in glutathione-depleted mice: structure-toxicity and metabolic studies[J]. Chem Res Toxicol, 2000, 13( 3): 170- 176. DOI: 10.1021/tx990155o. [11] HEIDARI R, BABAEI H, EGHBAL M. Mechanisms of methimazole cytotoxicity in isolated rat hepatocytes[J]. Drug Chem Toxicol, 2013, 36( 4): 403- 411. DOI: 10.3109/01480545.2012.749272. [12] SUN J, ZHAO F, LIN B, et al. Gut microbiota participates in antithyroid drug induced liver injury through the lipopolysaccharide related signaling pathway[J]. Front Pharmacol, 2020, 11: 598170. DOI: 10.3389/fphar.2020.598170. [13] CLARKE JI, DEAR JW, ANTOINE DJ. Recent advances in biomarkers and therapeutic interventions for hepatic drug safety-false dawn or new horizon?[J]. Expert Opin Drug Saf, 2016, 15( 5): 625- 634. DOI: 10.1517/14740338.2016.1160057. [14] DEAR JW, CLARKE JI, FRANCIS B, et al. Risk stratification after paracetamol overdose using mechanistic biomarkers: results from two prospective cohort studies[J]. Lancet Gastroenterol Hepatol, 2018, 3( 2): 104- 113. DOI: 10.1016/S2468-1253(17)30266-2. [15] GALLELLI L, STALTARI O, PALLERIA C, et al. Hepatotoxicity induced by methimazole in a previously healthy patient[J]. Curr Drug Saf, 2009, 4( 3): 204- 206. DOI: 10.2174/157488609789006912. [16] LI L, JIANG W, WANG J. Clinical analysis of 275 cases of acute drug-induced liver disease[J]. Front Med China, 2007, 1( 1): 58- 61. DOI: 10.1056/NEJMra052270. [17] FORD R, SCHWARTZ L, DANCEY J, et al. US Food and Drug Administration. Center for Drug Evaluation and Research(CDER), Center for Biologics Evaluation and Research(CBER). Guidance for industry: drug-induced liver injury-premarketing clinical evaluation[J]. Eur J Cancer, 2009, 45( 2): 268- 274. [18] de ALMEIDA R, MCCALMON S, CABANDUGAMA PK. Clinical review and update on the management of thyroid storm[J]. Mo Med, 2022, 119( 4): 366- 371. [19] WAN YM, WU JF, LI YH, et al. Prednisone is not beneficial for the treatment of severe drug-induced liver injury: An observational study(STROBE compliant)[J]. Medicine(Baltimore), 2019, 98( 26): e15886. DOI: 10.1097/MD.0000000000015886. [20] MA JGJ, LAMMERT C. Characterization of steroid therapy for drug-induced liver injury[J]. Gastroenterology, 2020, 158( 6): S-1304. DOI: 10.1016/S0016-5085(20)33922-6. [21] WANG JB, HUANG A, WANG Y, et al. Corticosteroid plus glycyrrhizin therapy for chronic drug- or herb-induced liver injury achieves biochemical and histological improvements: a randomised open-label trial[J]. Aliment Pharmacol Ther, 2022, 55( 10): 1297- 1310. DOI: 10.1111/apt.16902. [22] HEIDARI R, BABAEI H, ROSHANGAR L, et al. Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine[J]. Adv Pharm Bull, 2014, 4( 1): 21- 28. DOI: 10.5681/apb.2014.004. [23] SQUIRES RH, DHAWAN A, ALONSO E, et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial[J]. Hepatology, 2013, 57( 4): 1542- 1549. DOI: 10.1002/hep.26001. [24] YANG J, ZHONG J, ZHOU LZ, et al. Sudden onset agranulocytosis and hepatotoxicity after taking methimazole[J]. Intern Med, 2012, 51( 16): 2189- 2192. DOI: 10.2169/internalmedicine.51.7845. [25] WANG Y, WANG Z, GAO M, et al. Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: A phase II trial[J]. Liver Int, 2019, 39( 11): 2102- 2111. DOI: 10.1111/liv.14204. [26] LI WX, RAO Z, WU T, et al. Clinical effect of magnesium isoglycyrrhizinate in the treatment of drug-induced liver injury induced by methimazole[J]. Clin Ration Drug Use, 2023, 16( 16): 69- 71. DOI: 10.15887/j.cnki.13-1389/r.2023.16.019.李文旋, 饶志, 武涛, 等. 异甘草酸镁治疗甲巯咪唑致药物性肝损伤的临床效果[J]. 临床合理用药, 2023, 16( 16): 69- 71. DOI: 10.15887/j.cnki.13-1389/r.2023.16.019. [27] WANG Y, LAI R, ZONG P, et al. Bicyclol for the treatment of drug-induced liver injury: a propensity score matching analysis using a nationwide inpatient database[J]. J Int Med Res, 2021, 49( 4): 3000605211005945. DOI: 10.1177/03000605211005945. [28] TANG J, GU J, CHU N, et al. Efficacy and safety of bicyclol for treating patients with idiosyncratic acute drug-induced liver injury: A multicenter, randomized, phase II trial[J]. Liver Int, 2022, 42( 8): 1803- 1813. DOI: 10.1111/liv.15290. [29] APAYDıN T, ELBASAN O, YAVUZ DG. Preoperative plasmapheresis experience in Graves’ disease patients with anti-thyroid drug-induced hepatotoxicity[J]. Transfus Apher Sci, 2020, 59( 5): 102826. DOI: 10.1016/j.transci.2020.102826. [30] ZHANG Q, GUAN Y, XIANG T, et al. Combination of molecular adsorbent recirculating system and radioiodine for the treatment of concurrent hyperthyroidism and severe liver dysfunction: a retrospective cohort study[J]. Endocr Pract, 2017, 23( 2): 141- 148. DOI: 10.4158/EP161417.OR. [31] ZHENG M, CUI S, ZHANG W, et al. Graves’ disease overlapping with chronic hepatitis B and methimazole-induced liver injury and autoimmune hepatitis: a case report[J]. BMC Gastroenterol, 2022, 22( 1): 59. DOI: 10.1186/s12876-022-02133-z. [32] BENIĆ MS, NEŽIĆ L, VUJIĆ-ALEKSIĆ V, et al. Novel therapies for the treatment of drug-induced liver injury: a systematic review[J]. Front Pharmacol, 2021, 12: 785790. DOI: 10.3389/fphar.2021.785790. -



 PDF下载 ( 990 KB)
PDF下载 ( 990 KB)

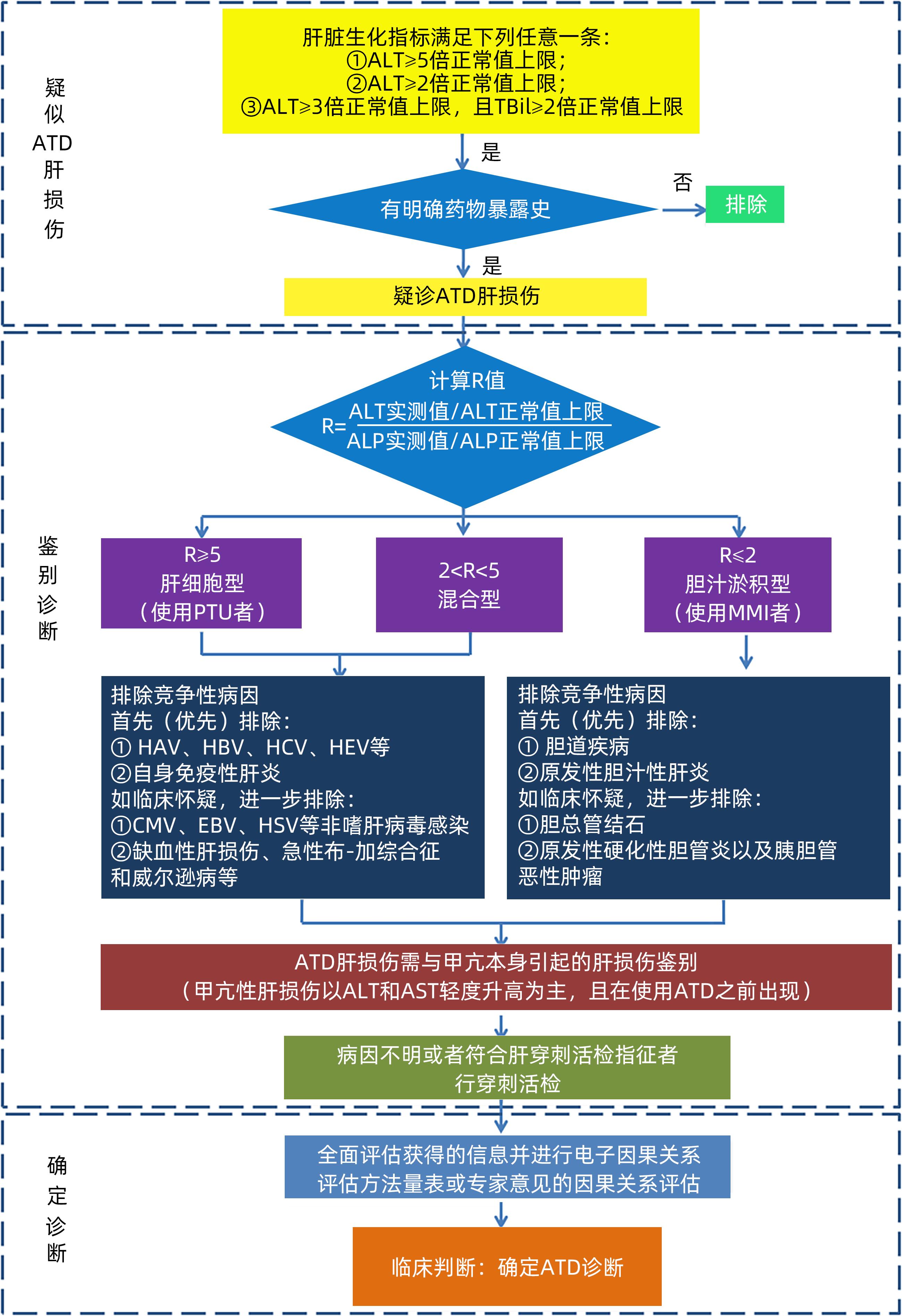
 下载:
下载:


