胰十二指肠切除术后胰瘘风险预测模型的建立: 基于2016新版胰瘘定义及分级系统
DOI: 10.12449/JCH240421
Establishment of a risk prediction model for pancreatic fistula after pancreaticoduodenectomy: A study based on the 2016 edition of the definition and classification system of pancreatic fistula
-
摘要:
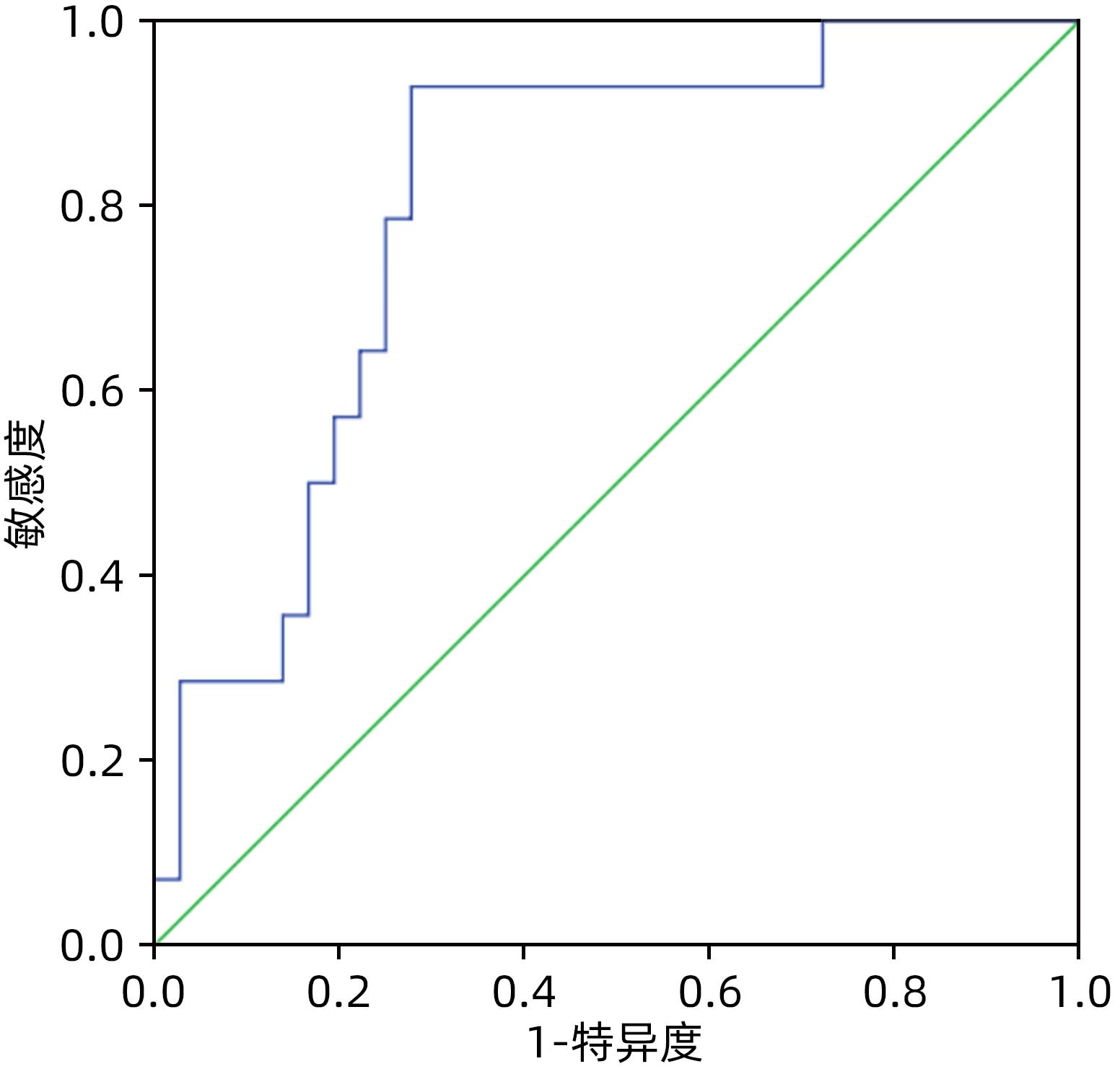
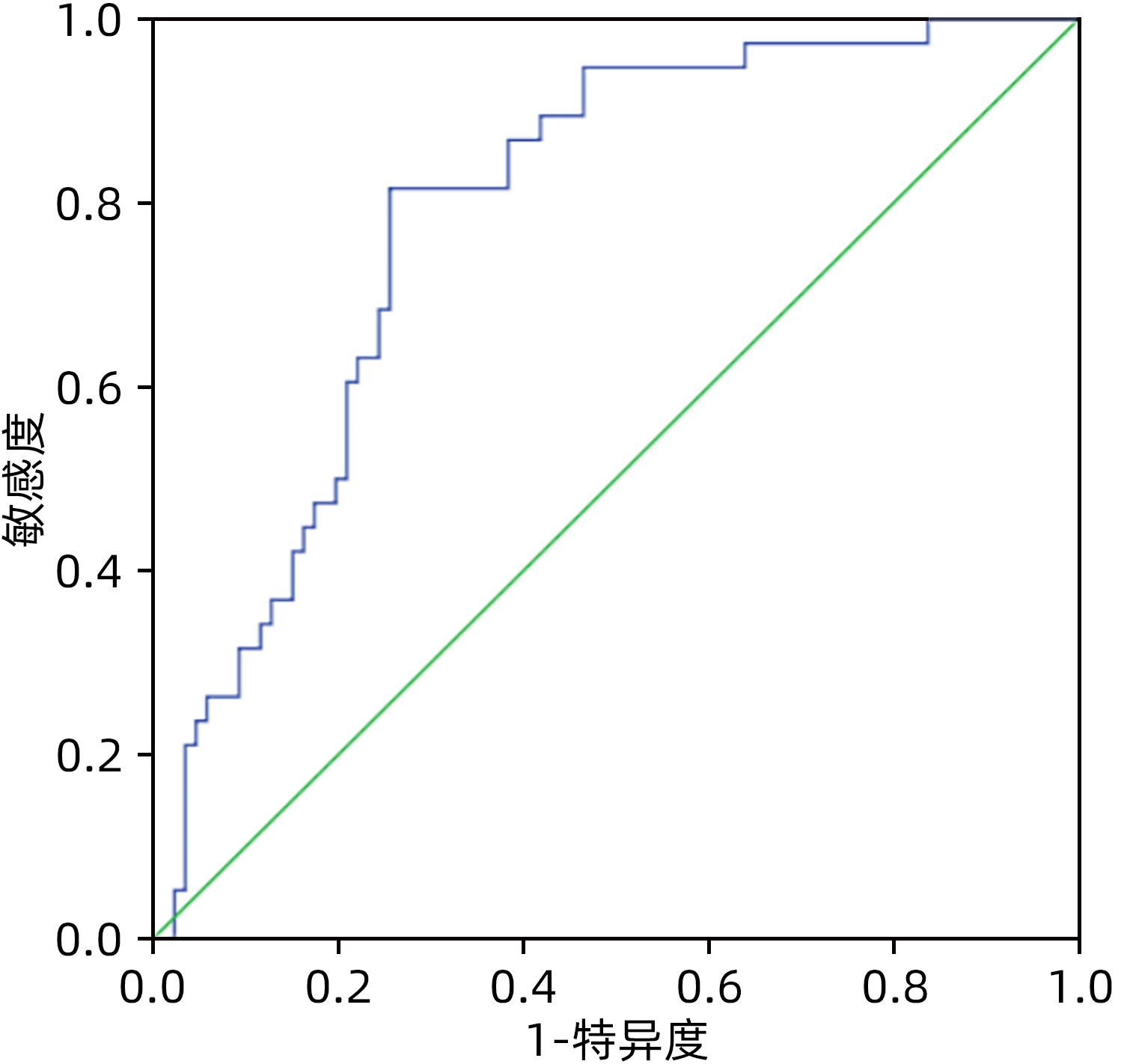
目的 比较分析2005版、2016版胰瘘定义及分级标准导致的胰十二指肠切除术(PD)术后胰瘘危险因素的差异,根据2016版胰瘘标准建立胰瘘风险预测模型。 方法 回顾性分析天津市第三中心医院2016年1月—2022年5月收治的303例行PD患者的临床资料,根据新、旧版胰瘘标准统计术后胰瘘患者,计量资料组间比较采用成组t检验或非参数检验Mann-Whitney U检验;计数资料组间比较采用χ2检验。单因素及多因素Logistic回归分析筛选两版标准对PD患者术后胰瘘的危险因素的区别,基于2016新版标准建立术后胰瘘的风险预测模型,受试者工作特征曲线分析该模型预测术后胰瘘发生的准确性并验证该模型。 结果 2005版胰瘘标准:单因素分析显示主胰管直径(χ2=31.641,P<0.001)、主胰管指数(χ2=52.777,P<0.001)、门静脉侵犯(χ2=6.259,P=0.012)、腹腔内脂肪厚度(χ2=7.665,P=0.006)、术前胆道引流(χ2=5.999,P=0.014)、胰腺癌(χ2=5.544,P=0.019)、切缘胰腺厚度(t=2.055,P=0.032)、胰腺CT值(t=-3.224,P=0.002)、术前血淀粉酶水平(Z=-2.099,P=0.036)与术后胰瘘的发生相关,Logistic回归分析显示主胰管指数[OR(95%CI)=0.000(0.000~0.011)]、胰腺癌[OR(95%CI)=4.843(1.285~18.254)]、胰腺CT值[OR(95%CI)=0.869(0.806~0.937)]为独立危险因素(P值均<0.05);而基于2016版胰瘘标准:单因素分析显示主胰管直径(χ2=5.391,P=0.020)、主胰管指数(χ2=11.394,P=0.001)、腹腔内脂肪厚度(χ2=8.899,P=0.003)、胰腺切缘厚度(t=2.665,P=0.009)、胰腺CT值(t=-2.835,P=0.004)与术后胰瘘的发生相关,Logistic回归分析显示主胰管指数[OR(95%CI)=0.001(0.000~0.050)]、胰腺CT值[OR(95%CI)=0.943(0.894~0.994)]为独立危险因素(P值均<0.05)。据此建立PD术后胰瘘风险预测模型,受试者工作特征曲线分析表明该模型预测PD术后胰瘘的曲线下面积在建模组与验证组分别为0.788(95%CI:0.707~0.870)和0.804(95%CI:0.675~0.932)。 结论 主胰管指数、胰腺CT值与PD术后胰瘘的发生密切相关,基于2016新版胰瘘标准建立的胰瘘风险预测模型具有较好的预测性能。 Abstract:Objective To investigate the differences in the risk factors for postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD) between the 2005 and 2016 editions of the definition and classification standards for pancreatic fistula, and to establish a risk prediction model for pancreatic fistula based on the 2016 edition. Methods A retrospective analysis was performed for the clinical data of 303 patients who were admitted to Tianjin Third Central Hospital and underwent PD from January 2016 to May 2022, and the patients with POPF were identified based on the new and old editions. The independent-samples t test or the non-parametric Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. The univariate and multivariate logistic regression analyses were used to investigate the differences in the risk factors for pancreatic fistula after PD between the two editions; a risk prediction model was established for POPF based on the 2016 edition, and the receiver operating characteristic curve was used to invesitgate the accuracy of this model in predicting POPF and perform model validation. Results According to the 2005 edition, the univariate analysis showed that the diameter of the main pancreatic duct (χ2=31.641, P<0.001), main pancreatic duct index (χ2=52.777, P<0.001), portal vein invasion (χ2=6.259, P=0.012), intra-abdominal fat thickness (χ2=7.665, P=0.006), preoperative biliary drainage (χ2=5.999, P=0.014), pancreatic cancer (χ2=5.544, P=0.019), marginal pancreatic thickness (t=2.055, P=0.032), pancreatic CT value (t=-3.224, P=0.002), and preoperative blood amylase level (Z=-2.099, P=0.036) were closely associated with POPF, and the multivariate logistic regression analysis showed that main pancreatic duct index (odds ratio [OR]=0.000, 95% confidence interval [CI]: 0.000 — 0.011, P<0.05), pancreatic cancer (OR=4.843, 95%CI: 1.285 — 18.254, P<0.05), and pancreatic CT value (OR=0.869, 95%CI: 0.806 — 0.937, P<0.05) were independent risk factors; based on the 2016 edition, the univariate analysis showed the diameter of the main pancreatic duct (χ2=5.391, P=0.020), main pancreatic duct index (χ2=11.394, P=0.001), intra-abdominal fat thickness (χ2=8.899, P=0.003), marginal pancreatic thickness (t=2.665, P=0.009), pancreatic CT value (t=-2.835, P=0.004) were closely associated with POPF, and the multivariate logistic regression analysis showed that main pancreatic duct index (OR=0.001, 95%CI: 0.000 — 0.050, P<0.05) and pancreatic CT value (OR=0.943, 95%CI: 0.894 — 0.994, P<0.05) were independent risk factors. A risk prediction model was established for POPF after PD, and the ROC curve analysis showed that this model had an area under the ROC curve of 0.788 (95%CI: 0.707 — 0.870) in the modeling group and 0.804 (95%CI: 0.675 — 0.932) in the validation group. Conclusion Main pancreatic duct index and pancreatic CT value are closely associated with POPF after PD, and the risk prediction model for pancreatic fistula based on the 2016 edition has a good prediction accuracy. -
Key words:
- Pancreaticoduodenectomy /
- Pancreatic Fistula /
- Pancreatic Ducts /
- Forecasting
-
表 1 2005版与2016版术后胰瘘定义与分级系统比较
Table 1. Comparison of definition and grading system for POPF between 2005 and 2016
2005年ISGPF版术后胰瘘定义与分级系统 分级 临床表现 特殊治疗 超声或CT 持续引流>3周 二次手术 胰瘘相关死亡 感染征象 脓毒症 再入院 A级 良好 无 阴性 无 无 无 无 无 无 B级 通常良好 有/无 阴性/阳性 通常有 无 无 有 无 有/无 C级 病容/差 有 阳性 有 有 可能有 有 有 有/无 2016年ISGPS版术后胰瘘定义与分级系统 定义及分级 术后第3天引流液的淀粉酶数值达正常上限的3倍以上 胰周持续引流>3周 临床相关的胰瘘治疗措施改变 经皮或内镜下穿刺引流 血管造影介入治疗术后胰瘘相关出血 二次手术 术后胰瘘相关的感染征象 术后胰瘘相关器官衰竭 术后胰瘘相关死亡 生化漏 有 无 无 无 无 无 无 无 无 B级 有 有 有 有 有 无 有(无器官衰竭) 无 无 C级 有 有 有 有 有 有 有 有 有 表 2 建模组与验证组患者的临床特征比较
Table 2. Clinical characteristics of the modeling and validation sets
指标 建模组(n=253) 验证组(n=50) P值 性别[例(%)] 0.416 男 167(66.0) 30(60.0) 女 86(34.0) 20(40.0) 年龄(岁) 61±9 62±7 0.686 BMI(kg/m2) 23.1±2.7 22.4±3.3 0.312 饮酒[例(%)] 0.246 是 48(19.0) 13(26.0) 否 205(81.0) 37(74.0) 吸烟[例(%)] 0.846 是 110(43.5) 21(42.0) 否 143(56.5) 29(58.0) 糖尿病[例(%)] 0.983 是 56(22.1) 11(22.0) 否 197(77.9) 39(78.0) 主胰管直径(mm) 3.8±2.1 3.7±2.2 0.606 切缘胰腺厚度(mm) 15.3±3.5 14.7±3.1 0.220 主胰管指数 0.3±0.2 0.3±0.2 0.885 门静脉受侵犯[例(%)] 0.901 是 16(6.3) 4(8.0) 否 237(93.7) 46(92.0) 腹腔内脂肪厚度(mm) 71.1±27.2 68.3±25.3 0.389 胰腺CT值(HU) 38.6±8.3 38.1±8.8 0.693 术前胆道引流[例(%)] 0.707 是 30(11.9) 5(10.0) 否 223(88.1) 45(90.0) 术前实验室检查 白细胞(×109/L) 6.1±1.7 5.9±1.6 0.397 血小板(×109/L) 232.5±68.5 240.3±62.8 0.316 白蛋白(g/L) 39.3±4.1 38.7±3.8 0.662 总胆红素(μmol/L) 121.3(34.1~231.4) 130.5(39.2~190.0) 0.747 血清淀粉酶(IU/L) 25.0(16.0~42.0) 26.0(19.5~39.3) 0.737 CA19-9(U/mL) 81.9(34.2~273.1) 94.0(28.5~357.5) 0.626 胰肠吻合方式[例(%)] 0.814 胰管空肠黏膜对黏膜 47(18.6) 10(20.0) 胰肠端侧套入式 206(81.4) 40(80.0) 胰腺癌[例(%)] 0.684 是 59(23.3) 13(26.0) 否 194(76.7) 37(74.0) 术后胰瘘[例(%)] 2005版ISGPF 0.517 是 124(49.0) 22(44.0) 否 129(51.0) 28(56.0) 2016版ISGPS 0.987 是 61(24.1) 12(24.0) 否 192(75.9) 38(76.0) 手术相关死亡[例(%)] 0.658 是 8(3.2) 1(2.0) 否 245(96.8) 49(98.0) 表 3 与PD术后胰瘘相关危险因素单因素分析结果(2005版ISGPF)
Table 3. Univariate analysis results of risk factors related to POPF after PD (2005 ISGPF edition)
指标 PD术后胰瘘 统计值 P值 有 无 主胰管直径(例) χ2=31.641 <0.001 ≤3 mm 88 46 >3 mm 36 83 主胰管指数(例) χ2=52.777 <0.001 ≤0.25 101 47 >0.25 23 82 门静脉受侵犯(例) χ2=6.259 0.012 有 3 13 无 121 116 腹腔内脂肪厚度(例) χ2=7.665 0.006 ≤65 mm 43 67 >65 mm 81 62 术前胆道引流(例) χ2=5.999 0.014 有 21 9 无 103 120 胰腺癌(例) χ2=5.544 0.019 是 21 38 否 103 91 切缘胰腺厚度(mm) 16.5±3.7 15.0±3.8 t=2.055 0.032 胰腺CT值(HU) 36.3±6.4 41.1±9.6 t=-3.224 0.002 性别(例) χ2=0.572 0.449 男 79 88 女 45 41 饮酒(例) χ2=1.242 0.265 有 27 21 无 97 108 吸烟(例) χ2=0.001 0.974 有 54 56 无 70 73 糖尿病(例) χ2=0.549 0.459 有 25 31 无 99 98 胰肠吻合方式(例) χ2=1.703 0.192 胰管空肠黏膜对黏膜 19 28 胰肠端侧套入式 105 101 年龄(岁) 61±9 61±7 t=-0.259 0.796 BMI(kg/m2) 23.0±3.1 23.2±2.8 t=-0.372 0.626 术前实验室检查 白细胞(×109/L) 6.1±1.9 6.0±1.6 t=0.773 0.441 血小板(×109/L) 230.4±69.6 235.0±64.1 t=-0.892 0.374 白蛋白(g/L) 39.4±4.2 38.9±4.1 t=0.757 0.450 总胆红素(μmol/L) 124.8(55.6~249.3) 117.8(37.0~219.1) U=-1.093 0.275 CA19-9(U/mL) 73.3(29.4~222.6) 96.2(42.0~370.1) U=-1.069 0.285 血清淀粉酶(IU/L) 23.0(17.0~34.0) 31.5(17.3~60.0) U=-2.099 0.036 表 4 与PD术后胰瘘相关危险因素单因素分析结果(2016版ISGPS)
Table 4. Univariate analysis results of risk factors related to POPF after PD (2016 ISGPS edition)
指标 PD术后胰瘘 统计值 P值 有 无 主胰管直径(例) χ2=5.391 0.020 ≤3 mm 39 90 >3 mm 22 102 主胰管指数(例) χ2=11.394 0.001 ≤0.25 47 101 >0.25 14 91 腹腔内脂肪厚度(例) χ2=8.899 0.003 ≤65 mm 16 92 >65 mm 45 100 切缘胰腺厚度(mm) 17.0±4.2 14.5±3.6 t=2.665 0.009 胰腺CT值(HU) 36.2±7.3 39.7±8.8 t=-2.835 0.004 门静脉受侵犯(例) χ2=0.672 0.412 有 2 14 无 59 178 术前胆道引流(例) χ2=2.933 0.087 有 11 19 无 50 173 胰腺癌(例) χ2=1.721 0.190 是 18 41 否 43 151 性别(例) χ2=0.720 0.396 男 43 124 女 18 68 饮酒(例) χ2=0.286 0.593 有 13 35 无 48 157 吸烟(例) χ2=0.020 0.887 有 27 83 无 34 109 糖尿病(例) χ2=0.283 0.595 有 12 44 无 49 148 胰肠吻合方式(例) χ2=2.680 0.102 胰管空肠黏膜对黏膜 7 40 胰肠端侧套入式 54 152 年龄(岁) 61±8 60±10 t=1.246 0.215 BMI(kg/m2) 22.9±4.3 23.3±2.1 t=-0.738 0.534 术前实验室检查 白细胞(×109/L) 6.2±2.3 6.0±1.7 t=0.474 0.673 血小板(×109/L) 230.9±67.9 237.8±73.2 t=-0.523 0.602 白蛋白(g/L) 39.2±4.3 39.0±4.0 t=0.303 0.762 总胆红素(μmol/L) 127.1(50.0~231.4) 121.5(32.9~216.6) U=-0.882 0.378 CA19-9(U/mL) 97.4(34.5~314.4) 80.9(27.3~344.3) U=-0.498 0.618 血清淀粉酶(IU/L) 24.0(17.0~33.8) 30.0(17.0~58.0) U=-1.818 0.069 表 5 与PD术后胰瘘相关危险因素多因素Logistic分析结果
Table 5. Multivariate analysis results of risk factors related to POPF after PD
指标 系数 OR值(95%CI) P值 2005版ISGPF 常量 6.248 517.032 <0.001 主胰管指数 -8.045 0.000(0.000~0.011) <0.001 是否胰腺癌 1.578 4.843(1.285~18.254) 0.020 胰腺CT值 -0.141 0.869(0.806~0.937) <0.001 2016版ISGPS 常量 2.667 14.393 0.018 主胰管指数 -6.995 0.001(0.000~0.050) 0.001 胰腺CT值 -0.059 0.943(0.894~0.994) 0.029 -
[1] LI Y, SHI YB, TU JH, et al. Risk factors and prophylaxis for pancreatic fistula after pancreaticoduodenectomy[J/OL]. Chin J Hepatic Surg(Electronic Edition), 2023, 12( 3): 352- 355. DOI: 10.3877/cma.j.issn.2095-3232.2023.03.021.李扬, 史亚波, 涂建华, 等. 胰十二指肠切除术后胰瘘发生的危险因素及预防[J/OL]. 中华肝脏外科手术学电子杂志, 2023, 12( 3): 352- 355. DOI: 10.3877/cma.j.issn.2095-3232.2023.03.021. [2] LIU L, XU ZH, WANG WQ, et al. Prevention and management of pancreatic fistula after pancreatoduodenectomy with precise and comprehensive opinion[J]. Chin J Dig Surg, 2023, 22( 5): 657- 662. DOI: 10.3760/cma.j.cn115610-20230401-00143.刘亮, 徐志航, 王文权, 等. 精准联合综合策略防治胰十二指肠切除术后胰瘘[J]. 中华消化外科杂志, 2023, 22( 5): 657- 662. DOI: 10.3760/cma.j.cn115610-20230401-00143. [3] BASSI C, BUCHLER MW, FINGERHUT A, et al. Predictive factors for postoperative pancreatic fistula[J]. Ann Surg, 2015, 261( 4): e99. DOI: 10.1097/SLA.0000000000000577. [4] MALGRAS B, DOKMAK S, AUSSILHOU B, et al. Management of postoperative pancreatic fistula after pancreaticoduodenectomy[J]. J Visc Surg, 2023, 160( 1): 39- 51. DOI: 10.1016/j.jviscsurg.2023.01.002. [5] AOKI S, MIYATA H, KONNO H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: A nationwide study of 17, 564 patients in Japan[J]. J Hepatobiliary Pancreat Sci, 2017, 24( 5): 243- 251. DOI: 10.1002/jhbp.438. [6] BASSI C, DERVENIS C, BUTTURINI G, et al. Postoperative pancreatic fistula: An international study group(ISGPF) definition[J]. Surgery, 2005, 138( 1): 8- 13. DOI: 10.1016/j.surg.2005.05.001. [7] BASSI C, MARCHEGIANI G, DERVENIS C, et al. The 2016 update of the International Study Group(ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After[J]. Surgery, 2017, 161( 3): 584- 591. DOI: 10.1016/j.surg.2016.11.014. [8] CHEN HP, SHAO WX, LONG DY. Value of preoperative computed tomography for prediction of pancreatic fistula after pancreaticoduodenectomy[J]. World Chin J Dig, 2015, 23( 9): 1489- 1494. DOI: 10.11569/wcjd.v23.i9.1489.陈和平, 邵伟新, 龙德云. 手术前计算机断层扫描对胰十二指肠切除术后患者发生胰瘘的预测价值[J]. 世界华人消化杂志, 2015, 23( 9): 1489- 1494. DOI: 10.11569/wcjd.v23.i9.1489. [9] WENG H, SHU YJ, BAO RF, et al. Preoperative pancreas plain scan CT value for the evaluation of the risk of postoperative pancreatic fistula[J]. Chin J Gen Surg, 2014, 29( 1): 21- 24. DOI: 10.3760/cma.j.issn.1007-631X.2014.01.006.翁昊, 束翌俊, 包润发, 等. 术前胰腺平扫CT值可预测胰十二指肠切除术后胰瘘危险性[J]. 中华普通外科杂志, 2014, 29( 1): 21- 24. DOI: 10.3760/cma.j.issn.1007-631X.2014.01.006. [10] SHI S, XIANG JF, XU J, et al. Updates and interpretations of PODF definition and grading system(2016 edition) by ISGPS[J]. Chin J Pract Surg, 2017, 37( 2): 149- 152. DOI: 10.19538/j.cjps.issn1005-2208.2017.02.12.施思, 项金峰, 徐近, 等. 2016版 国际胰腺外科研究组术后胰瘘定义和分级系统更新内容介绍和解析[J]. 中国实用外科杂志, 2017, 37( 2): 149- 152. DOI: 10.19538/j.cjps.issn1005-2208.2017.02.12. [11] WU JZ, TANG ZQ, ZHAO G, et al. Incidence and risk factors for postoperative pancreatic fistula in 2089 patients treated by radical gastrectomy: A prospective multicenter cohort study in China[J]. Int J Surg, 2022, 98: 106219. DOI: 10.1016/j.ijsu.2021.106219. [12] HALLE-SMITH JM, VINUELA E, BROWN RM, et al. A comparative study of risk factors for pancreatic fistula after pancreatoduodenectomy or distal pancreatectomy[J]. HPB, 2017, 19( 8): 727- 734. DOI: 10.1016/j.hpb.2017.04.013. [13] POTTER KC, SUTTON TL, O’GRADY J, et al. Risk factors for postoperative pancreatic fistula in the Era of pasireotide[J]. Am J Surg, 2022, 224( 2): 733- 736. DOI: 10.1016/j.amjsurg.2022.02.050. [14] ROBERTS KJ, SUTCLIFFE RP, MARUDANAYAGAM R, et al. Scoring system to predict pancreatic fistula after pancreaticoduodenectomy: A UK multicenter study[J]. Ann Surg, 2015, 261( 6): 1191- 1197. DOI: 10.1097/SLA.0000000000000997. [15] SIMON R. Complications after pancreaticoduodenectomy[J]. Surg Clin North Am, 2021, 101( 5): 865- 874. DOI: 10.1016/j.suc.2021.06.011. [16] WADA K, TRAVERSO LW. Pancreatic anastomotic leak after the Whipple procedure is reduced using the surgical microscope[J]. Surgery, 2006, 139( 6): 735- 742. DOI: 10.1016/j.surg.2005.11.001. [17] SUGIMOTO M, TAKAHASHI S, KOJIMA M, et al. In patients with a soft pancreas, a thick parenchyma, a small duct, and fatty infiltration are significant risks for pancreatic fistula after pancreaticoduodenectomy[J]. J Gastrointest Surg, 2017, 21( 5): 846- 854. DOI: 10.1007/s11605-017-3356-7. [18] AKAMATSU N, SUGAWARA Y, KOMAGOME M, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: The significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage[J]. J Hepato Biliary Pancreat Sci, 2010, 17( 3): 322- 328. DOI: 10.1007/s00534-009-0248-6. [19] CHEN JY, FENG J, WANG XQ, et al. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy[J]. World J Gastroenterol, 2015, 21( 19): 5926- 5933. DOI: 10.3748/wjg.v21.i19.5926. [20] KAMARAJAH SK, BUNDRED JR, LIN A, et al. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy[J]. ANZ J Surg, 2021, 91( 5): 810- 821. DOI: 10.1111/ans.16408. [21] UTSUMI M, AOKI H, NAGAHISA S, et al. Preoperative predictive factors of pancreatic fistula after pancreaticoduodenectomy: Usefulness of the CONUT score[J]. Ann Surg Treat Res, 2020, 99( 1): 18- 25. DOI: 10.4174/astr.2020.99.1.18. [22] KAJIWARA T, SAKAMOTO Y, MOROFUJI N, et al. An analysis of risk factors for pancreatic fistula after pancreaticoduodenectomy: Clinical impact of bile juice infection on day 1[J]. Langenbecks Arch Surg, 2010, 395( 6): 707- 712. DOI: 10.1007/s00423-009-0547-z. [23] ADAMU M, PLODECK V, ADAM C, et al. Predicting postoperative pancreatic fistula in pancreatic head resections: Which score fits all?[J]. Langenbecks Arch Surg, 2022, 407( 1): 175- 188. DOI: 10.1007/s00423-021-02290-x. [24] TRANCHART H, GAUJOUX S, REBOURS V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy[J]. Ann Surg, 2012, 256( 1): 139- 145. DOI: 10.1097/SLA.0b013e318256c32c. [25] JANG M, PARK HW, HUH J, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI[J]. Eur Radiol, 2019, 29( 5): 2417- 2425. DOI: 10.1007/s00330-018-5790-7. [26] WENG H, FAN QQ, SONG XL, et al. Preoperative pancreatic CT value is related to pancreatic fistula after pancreaticoduodenectomy: A retrospective study[J]. Gland Surg, 2023, 12( 2): 243- 251. DOI: 10.21037/gs-23-19. [27] CALLERY MP, PRATT WB, KENT TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy[J]. J Am Coll Surg, 2013, 216( 1): 1- 14. DOI: 10.1016/j.jamcollsurg.2012.09.002. [28] ROBERTS KJ, HODSON J, MEHRZAD H, et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy[J]. HPB, 2014, 16( 7): 620- 628. DOI: 10.1111/hpb.12186. [29] CHEN YR, TIAN XD, XIE XH, et al. Risk factors of postoperative pancreatic fistula after pancreaticoduodenectomy and its predictive score[J]. Chin J Surg, 2016, 54( 1): 39- 43. DOI: 10.3760/cma.j.issn.0529-5815.2016.01.010.陈依然, 田孝东, 谢学海, 等. 胰十二指肠切除术后胰瘘风险预测系统的建立和应用[J]. 中华外科杂志, 2016, 54( 1): 39- 43. DOI: 10.3760/cma.j.issn.0529-5815.2016.01.010. [30] MUNGROOP TH, van RIJSSEN LB, van KLAVEREN D, et al. Alternative fistula risk score for pancreatoduodenectomy(a-FRS): Design and international external validation[J]. Ann Surg, 2019, 269( 5): 937- 943. DOI: 10.1097/SLA.0000000000002620. [31] XU XB, JIA CP, JIA Y, et al. Construction and application value of prediction model of pancreatic fistula after pancreaticoduodenectomy[J]. Chin J Dig Surg, 2020, 19( 4): 408- 413. DOI: 10.3760/cma.j.cn115610-20200409-00240.徐西伯, 贾成朋, 贾勇, 等. 构建胰十二指肠切除术后胰瘘预测模型及其应用价值[J]. 中华消化外科杂志, 2020, 19( 4): 408- 413. DOI: 10.3760/cma.j.cn115610-20200409-00240. [32] GUILBAUD T, GARNIER J, GIRARD E, et al. Postoperative day 1 combination of serum C-reactive protein and drain amylase values predicts risks of clinically relevant pancreatic fistula. The“90-1000” score[J]. Surgery, 2021, 170( 5): 1508- 1516. DOI: 10.1016/j.surg.2021.04.033. -



 PDF下载 ( 797 KB)
PDF下载 ( 797 KB)

 下载:
下载: