低碳水化合物饮食和生活方式干预对瘦型非酒精性脂肪性肝病患者的疗效观察
DOI: 10.12449/JCH240513
Therapeutic effect of low-carbohydrate diet and lifestyle intervention on patients with lean nonalcoholic fatty liver disease
-
摘要:
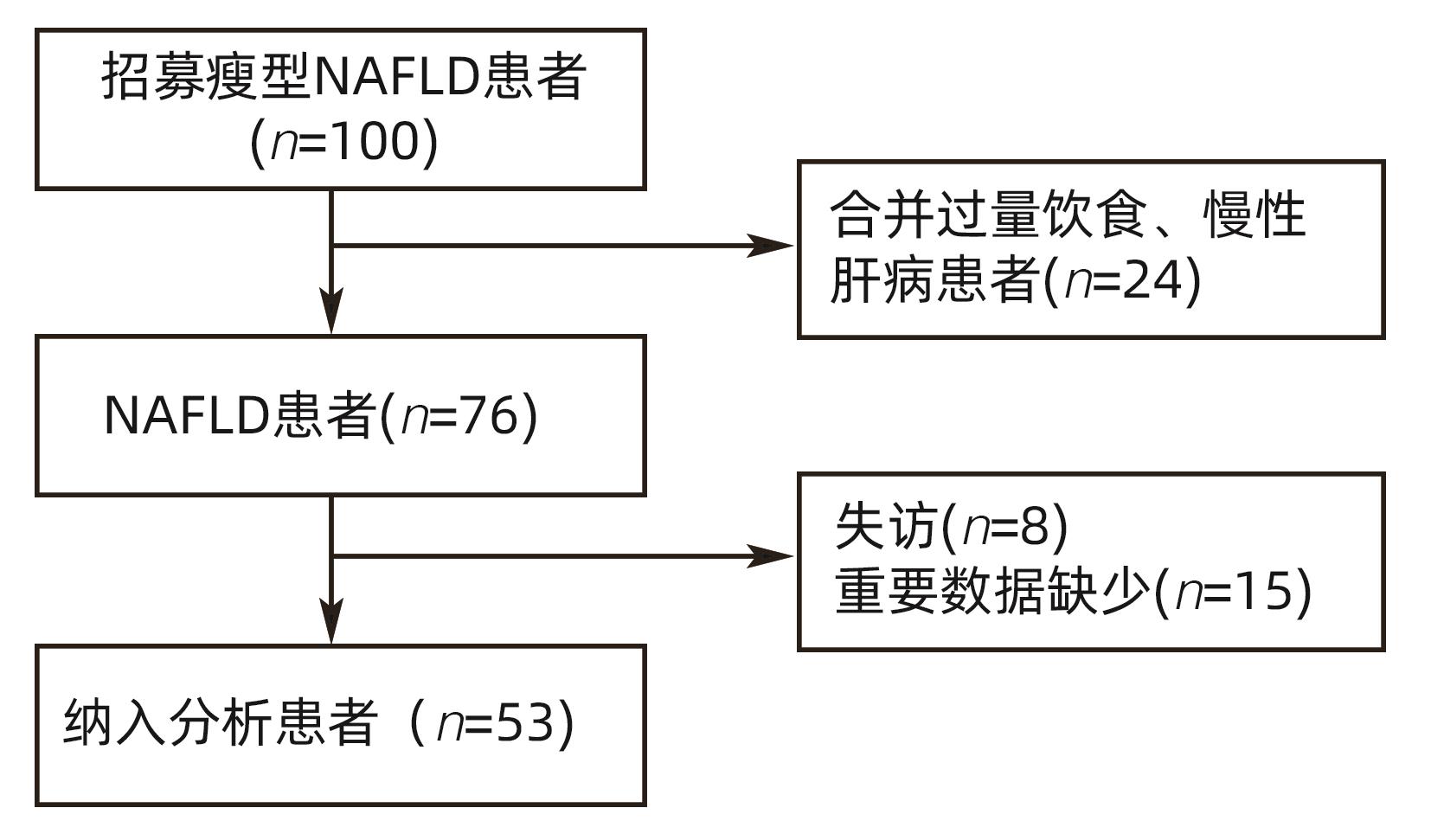
目的 观察低碳水化合物饮食和线上生活方式干预对痩型非酒精性脂肪性肝病(NAFLD)患者的疗效。 方法 本研究选取2019年12月—2021年3月在北京大学深圳医院感染性疾病科、深圳市前海蛇口自贸区医院感染性疾病科就诊的痩型NAFLD患者53例,予以限制热量摄入的低碳水化合物饮食[总热量摄入根据基础代谢率(BMR)和活动因子(PAL)计算得来,总能量限制在(BMR×95%×PAL-1 000)kcal~(BMR×95%×PAL-500)kcal],碳水化合物比例波动在10%~55%和生活方式指导8周,通过线上管理软件进行监督随访并观察患者疗效及安全性。比较患者干预前后的脂肪含量(CAP)及肝硬度(LSM)、人体测量学指标、血生化、尿蛋白和尿酮体等。1年后随访患者体质量和BMI。符合正态分布的计量资料两组间比较采用成组t检验;不符合正态分布的计量资料采用配对样本的Wilcoxon符号秩和检验。计数资料组间比较采用χ2检验。 结果 经8周干预后,患者CAP从(304.47±31.91)db/m下降至(242.43±26.74)db/m,LSM从(7.43±2.41)kPa下降至(6.36±1.79)kPa,体质量从(64.29±7.37)kg下降至(60.24±7.08),差异均有统计学意义(t值分别为11.25、3.72、14.07,P值均<0.001)。25例患者(47.2%)脂肪肝消失,12例LSM异常者(63.2%)恢复正常。52例患者(98.1%)体质量平均下降(4.05±2.32)kg。CAP下降等级随着体质量下降幅度的增加而增加。经干预后患者BMI、腰围、臀围、ALT、AST、GGT、尿酸、空腹血糖、TG、TC、LDL显著下降,HDL显著升高(t值分别为12.85,13.77,10.28,7.64,6.21,8.35,6.83,6.31,7.4,4.97,5.95,-2.21,P值均<0.05)。ALT、AST、GGT、尿酸、空腹血糖、TG、TC、LDL基线异常者恢复正常的比例分别为75%、100%、81.8%、57.1%、100%、66.7%、73.5%、85.3%。尿素氮、血肌酐、尿蛋白、尿酮体无明显改变(P值均>0.05)。随访1年后患者体质量及BMI无反弹(P值均>0.05)。干预及随访期间患者无胃肠道反应。 结论 低碳水化合物饮食及生活方式干预,可以改善痩型NAFLD患者肝脂肪含量及肝功能、血脂指标,且安全性良好。 -
关键词:
- 非酒精性脂肪性肝病 /
- 膳食, 低碳水化合物 /
- 生活方式 /
- 膳食, 减重
Abstract:Objective To investigate the therapeutic effect of low-carbohydrate diet and online lifestyle intervention on patients with lean nonalcoholic fatty liver disease (NAFLD). Methods This study was conducted among 53 patients with lean NAFLD who attended Department of Infectious Diseases in Peking University Shenzhen Hospital and Shenzhen Qianhai Shekou Free Trade Zone Hospital from December 2019 to March 2021, and the patients were given low-carbohydrate diet for calorie restriction [total calorie intake was calculated based on basal metabolic rate (BMR) and physical activity level (PAL) and was restricted within (BMR×95%×PAL-1 000) kcal to (BMR×95%×PAL-500) kcal, and carbohydrate ratio fluctuated between 10% and 55%] and lifestyle interventions for 8 weeks. An online software was used for supervision and follow-up, and the patients were observed in terms of treatment outcome and safety. The patients were compared in terms of controlled attenuation parameter (CAP), liver stiffness measurement (LSM), Anthropometric parameters, blood biochemistry, urinary protein, and urine ketone body before and after intervention. The patients were followed up after 1 year to measure body weight and body mass index (BMI). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the paired-sample Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous; the chi-square test was used for comparison of categorical data between groups. Results After 8 weeks of intervention, CAP decreased from 304.47±31.91 db/m to 242.43±26.74 db/m, LSM decreased from 7.43±2.41 kPa to 6.36±1.79 kPa, and body weight decreased from 64.29±7.37 kg to 60.24±7.08 kg (t=11.25,3.72, and 14.07, all P<0.001). Of all patients, 25 (47.2%) had disappearance of fatty liver, and abnormal LSM in 12 patients (63.2%) returned to normal; 52 patients (98.1%) had a mean reduction of 4.05±2.32 kg in body weight. The degree of reduction in CAP increased with the degree of reduction in body weight. After intervention, there were significant reductions in BMI, waist circumference, hip circumference, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), uric acid, fasting blood glucose, triglyceride (TG), total cholesterol (TC), and low-density lipoprotein (LDL) and a significant increase in high-density lipoprotein (t=12.85, 13.77, 10.28, 7.64, 6.21, 8.35, 6.83, 6.31, 7.4, 4.97, 5.95, and -2.21, all P<0.05). The patients with abnormal ALT, AST, GGT, uric acid, fasting blood glucose, TG, TC, and LDL at baseline which returned to normal after intervention accounted for 75%, 100%, 81.8%, 57.1%, 100%, 66.7%, 73.5%, and 85.3%, respectively. There were no significant changes in blood urea nitrogen, serum creatinine, urine protein, and urine ketone body (all P>0.05). There was no rebound in body weight and BMI after 1 year of follow-up (P>0.05). There were no gastrointestinal reactions during intervention or follow-up. Conclusion Low-carbohydrate diet and lifestyle intervention can improve liver fat content, liver function, and blood lipid parameters in patients with lean NAFLD, with good safety. -
表 1 世界卫生组织预测BMR公式
Table 1. Formula for predicting basal metabolic rate by WHO
年龄 男性(kcal/d) 女性(kcal/d) 18~30岁 15.057×体质量+692.200 14.818×体质量+486.600 30~60岁 11.472×体质量+873.100 8.126×体质量+845.600 表 2 根据生活方式及习惯性体力活动进行PAL分级
Table 2. PAL classification based on lifestyle and habitual physical activity
分类 PAL 静态、轻体力活动生活方式 1.40~1.69 活跃、中度体力活动生活方式 1.70~1.99 剧烈、重体力活动生活方式 2.00~2.40 注:PAL为日常活动评分。 表 3 干预前后患者人体测量血指标及血生化变化情况
Table 3. Anthropometric measurement of blood indicators and blood biochemical changes
指标 干预前 干预后 t值 P值 体质量(kg) 64.29±7.37 60.24±7.08 14.07 <0.001 BMI(kg/m2) 22.56±0.98 21.16±1.07 12.85 <0.001 WC(cm) 86.57±5.75 82.91±4.82 13.77 <0.001 HC(cm) 94.36±5.04 91.79±4.40 10.28 <0.001 LSM(kPa) 7.43±2.41 6.36±1.79 3.72 <0.001 CAP(db/m) 304.47±31.91 242.43±26.74 11.25 <0.001 ALT(U/L) 63.11±27.34 35.28±13.01 7.64 <0.001 AST(U/L) 43.34±19.41 27.51±6.16 6.21 <0.001 GGT(U/L) 49.53±19.69 31.79±13.11 8.35 <0.001 UA(μmol/L) 475.40±99.17 394.70±79.13 6.83 <0.001 FBG(mmol/L) 5.65±0.86 5.00±0.61 6.31 <0.001 TG(mmol/L) 2.61±1.25 1.57±0.65 7.40 <0.001 TC(mmol/L) 5.83±0.97 5.16±0.66 4.97 <0.001 HDL(mmol/L 1.27±0.58 1.46±0.38 -2.21 0.032 LDL(mmol/L) 3.94±1.00 3.11±0.57 5.95 <0.001 BUN(mmol/L) 4.86±1.02 4.92±0.96 -0.30 0.766 Cr(μmol/L) 69.34±16.34 70.11±18.12 -0.23 0.823 表 4 患者体质量下降情况
Table 4. The degree of weight loss
体质量下降比例 <3% 3%~5% 5%~7% 7%~10% >10% 例(%) 5(9.4) 18(34.0) 10(18.9) 13(24.5) 6(11.3) 表 5 体质量下降和CAP下降的关系
Table 5. Relationship between percent weight loss and percent CAP loss
组别 例数 CAP下降<15%[例(%)] CAP下降15%~30%[例(%)] CAP下降>30%[例(%)] 体质量下降<5% 23 19(35.8) 10(18.9) 0(0.0) 体质量下降5%~10% 24 4(7.5) 11(20.8) 0(0.0) 体质量下降>10% 6 1(1.9) 5(9.4) 3(5.7) -
[1] YE Q, ZOU BY, YEO YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2020, 5( 8): 739- 752. DOI: 10.1016/S2468-1253(20)30077-7. [2] A RH, JIA HY, DING YH, et al. Research advances in nonalcoholic fatty liver disease in lean individuals[J]. J Clin Hepatol, 2020, 36( 5): 1154- 1159. DOI: 10.3969/j.issn.1001-5256.2020.05. 046.阿儒汗, 贾海燕, 丁艳华, 等. 瘦型非酒精性脂肪性肝病的研究进展[J]. 临床肝胆病杂志, 2020, 36( 5): 1154- 1159. DOI: 10.3969/j.issn.1001-5256.2020.05.046. [3] DELA CRUZ AC, BUGIANESI E, GEORGE J, et al. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2014, 146( 5): S909. DOI: 10.1016/s0016-5085(14)63307-2. [4] YOUNES R, GOVAERE O, PETTA S, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: Time for reappraisal of BMI-driven approach?[J]. Gut, 2022, 71( 2): 382- 390. DOI: 10.1136/gutjnl-2020-322564. [5] HAGSTRÖM H, NASR P, EKSTEDT M, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study[J]. Hepatol Commun, 2018, 2( 1): 48- 57. DOI: 10.1002/hep4.1124. [6] WANG QY, YOU H, OU XJ, et al. Non-obese histologically confirmed NASH patients with abnormal liver biochemistry have more advanced fibrosis[J]. Hepatol Int, 2019, 13( 6): 766- 776. DOI: 10.1007/s12072-019-09982-z. [7] LI CL, GUO PP, OKEKUNLE AP, et al. Lean non-alcoholic fatty liver disease patients had comparable total caloric, carbohydrate, protein, fat, iron, sleep duration and overtime work as obese non-alcoholic fatty liver disease patients[J]. J Gastroenterol Hepatol, 2019, 34( 1): 256- 262. DOI: 10.1111/jgh.14360. [8] SCHWARZ JM, LINFOOT P, DARE D, et al. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets[J]. Am J Clin Nutr, 2003, 77( 1): 43- 50. DOI: 10.1093/ajcn/77.1.43. [9] DONNELLY KL, SMITH CI, SCHWARZENBERG SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease[J]. J Clin Invest, 2005, 115( 5): 1343- 1351. DOI: 10.1172/JCI23621. [10] XIA Y. Dietary patterns associated with non-alcoholic fatty liver disease in Tianjin population: a case-control study[C]// The 12th National Nutrition Science Conference, Beijing, 2015.夏阳. 膳食模式在天津人群中与非酒精性脂肪肝关联:病例-对照研究[C]// 第十二届全国营养科学大会, 北京, 2015. [11] Fatty Liver Expert Committee, Chinese Medical Doctor Association, National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association. Guidelines of prevention and treatment for alcoholic liver disease: a 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 939- 946. DOI: 10.3969/j.issn.1001-5256.2018.05.006.中国医师协会脂肪性肝病专家委员会, 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 酒精性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 939- 946. DOI: 10.3969/j.issn.1001-5256.2018.05.006. [12] ZHAO C, LI MF, XIAO W, et al. Selection of calculation methods for daily total calories of overweight and obese adults[J]. Guangdong Med J, 2004, 25( 5): 577- 578. DOI: 10.13820/j.cnki.gdyx.2004.05.064.赵超, 黎梅芳, 肖文, 等. 成人超重和肥胖症患者每天总热量计算方法的选择[J]. 广东医学, 2004, 25( 5): 577- 578. DOI: 10.13820/j.cnki.gdyx.2004.05.064. [13] LIANG J, JIANG ZQ, HE YM, et al. Estimated equations of basal metabolic rate in Chinese healthy adults[J]. Chin J Sch Dr, 2008, 22( 4): 372- 374. DOI: 10.3969/j.issn.1001-7062.2008.04.002.梁洁, 蒋卓勤, 何玉敏, 等. 中国健康成人基础代谢率估算公式的探讨[J]. 中国校医, 2008, 22( 4): 372- 374. DOI: 10.3969/j.issn.1001-7062.2008.04.002. [14] YAO M, MCCRORY MA, MA G, et al. Energy requirements of urban Chinese adults with manual or sedentary occupations, determined using the doubly labeled water method[J]. Eur J Clin Nutr, 2002, 56( 7): 575- 584. DOI: 10.1038/sj.ejcn.1601361. [15] MA LC. Analysis of the intervention effect of low-carb diet on patients with metabolic-related fatty liver disease[D]. Yan’an: Yan’an University, 2021.马立翠. 低碳水化合物饮食对MAFLD患者的干预效果分析[D]. 延安: 延安大学, 2021. [16] WU LX, WANG WJ, TANG X, et al. Diagnostic value of FibroTouch controlled attenuation parameters for hepatic steatosis in NAFLD patients[J]. J New Chin Med, 2019, 50( 8): 579- 583. DOI: 10.3969/j.issn.0253-9802.2019.08.004.吴李贤, 王文佳, 汤曦, 等. FibroTouch受控衰减参数对NAFLD肝脏脂肪变性诊断价值[J]. 新医学, 2019, 50( 8): 579- 583. DOI: 10.3969/j.issn.0253-9802.2019.08.004. [17] SHEN F, ZHENG RD, MI YQ, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients[J]. World J Gastroenterol, 2014, 20( 16): 4702- 4711. DOI: 10.3748/wjg.v20.i16.4702. [18] SASSO M, BEAUGRAND M, de LEDINGHEN V, et al. Controlled attenuation parameter(CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes[J]. Ultrasound Med Biol, 2010, 36( 11): 1825- 1835. DOI: 10.1016/j.ultrasmedbio.2010.07.005. [19] CHEN JN, CHEN AP, PAN Q, et al. Influential factors and clinical value of controlled attenuation parameters in the evaluation of hepatic steatosis using FibroScan[J]. Chin Hepatol, 2016, 21( 10): 805- 809. DOI: 10.14000/j.cnki.issn.1008-1704.2016.10.001.陈建能, 陈爱萍, 潘勤, 等. FibroScan实施受控衰减参数检测肝脂肪变的影响因素及应用价值分析[J]. 肝脏, 2016, 21( 10): 805- 809. DOI: 10.14000/j.cnki.issn.1008-1704.2016.10.001. [20] KIM NH, KIM JH, KIM YJ, et al. Clinical and metabolic factors associated with development and regression of nonalcoholic fatty liver disease in nonobese subjects[J]. Liver Int, 2014, 34( 4): 604- 611. DOI: 10.1111/liv.12454. [21] ISOURA Y, CHO Y, FUJIMOTO H, et al. Effects of obesity reduction on transient elastography-based parameters in pediatric non-alcoholic fatty liver disease[J]. Obes Res Clin Pract, 2020, 14( 5): 473- 478. DOI: 10.1016/j.orcp.2020.08.005. [22] WANG Y, ZHENG JF. Research progress of nutritional intervention in nonalcoholic fatty liver disease[J]. J Med Postgrad, 2014, 27( 1): 99- 101. DOI: 10.16571/j.cnki.1008-8199.2014.01.012.王宇, 郑锦锋. 非酒精性脂肪肝营养干预的研究进展[J]. 医学研究生学报, 2014, 27( 1): 99- 101. DOI: 10.16571/j.cnki.1008-8199.2014.01.012. [23] MOORE MP, CUNNINGHAM RP, DASHEK RJ, et al. A fad too far? dietary strategies for the prevention and treatment of NAFLD[J]. Obesity, 2020, 28( 10): 1843- 1852. DOI: 10.1002/oby.22964. [24] SANTESSO N, AKL EA, BIANCHI M, et al. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis[J]. Eur J Clin Nutr, 2012, 66( 7): 780- 788. DOI: 10.1038/ejcn.2012.37. [25] WANG Y, JIANG MX, XU Q, et al. Effects of low-carbohydrate diet on obese adults with nonalcoholic fatty liver disease[J]. Mil Med J Southeast China, 2015, 17( 1): 5- 7, 25. DOI: 10.3969/j.issn.1672-271X.2015.01.002.王宇, 姜明霞, 许琦, 等. 低碳水化合物饮食对非酒精性脂肪肝肥胖患者的影响[J]. 东南国防医药, 2015, 17( 1): 5- 7, 25. DOI: 10.3969/j.issn.1672-271X.2015.01.002. [26] FAN JG, CAO HX. Role of diet and nutritional management in non-alcoholic fatty liver disease[J]. J Gastroenterol Hepatol, 2013, 28( Suppl 4): 81- 87. DOI: 10.1111/jgh.12244. [27] WANG CE, XU WT, GONG J, et al. Research progress in treatment of nonalcoholic fatty liver disease[J]. Clin J Med Offic, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06.王彩娥, 许文涛, 宫建, 等. 非酒精性脂肪性肝病治疗研究进展[J]. 临床军医杂志, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06. [28] MOYER VA, U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2012, 157( 5): 373- 378. DOI: 10.7326/0003-4819-157-5-201209040-00475. -



 PDF下载 ( 837 KB)
PDF下载 ( 837 KB)

 下载:
下载:


