内质网应激蛋白激酶RNA样ER激酶(PERK)通路对肝星状细胞激活及Ⅰ型胶原蛋白表达的影响
DOI: 10.12449/JCH240516
Effect of the protein kinase RNA-like endoplasmic reticulum kinase pathway in endoplasmic reticulum stress on hepatic stellate cell activation and collagen type I expression
-
摘要:
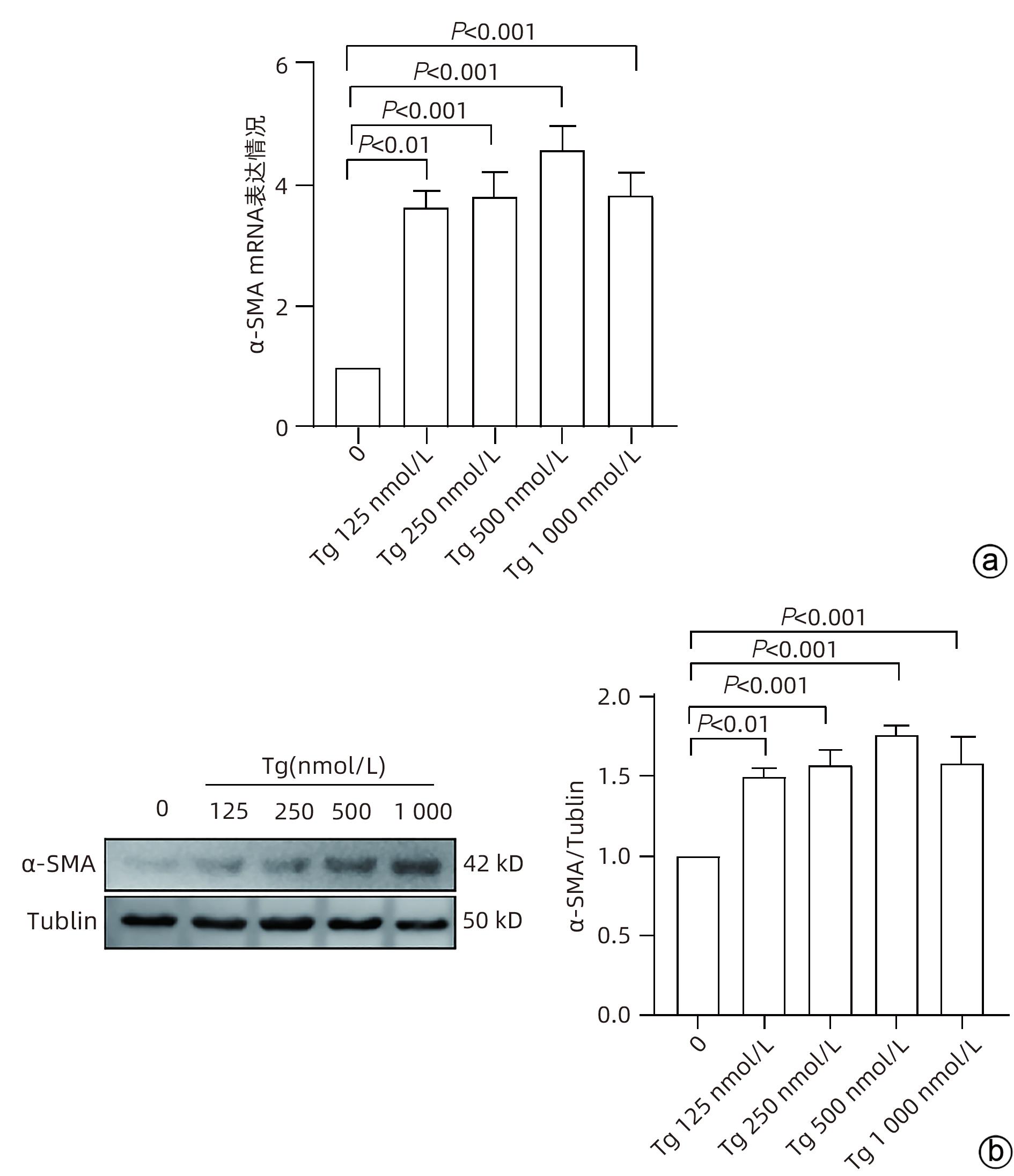
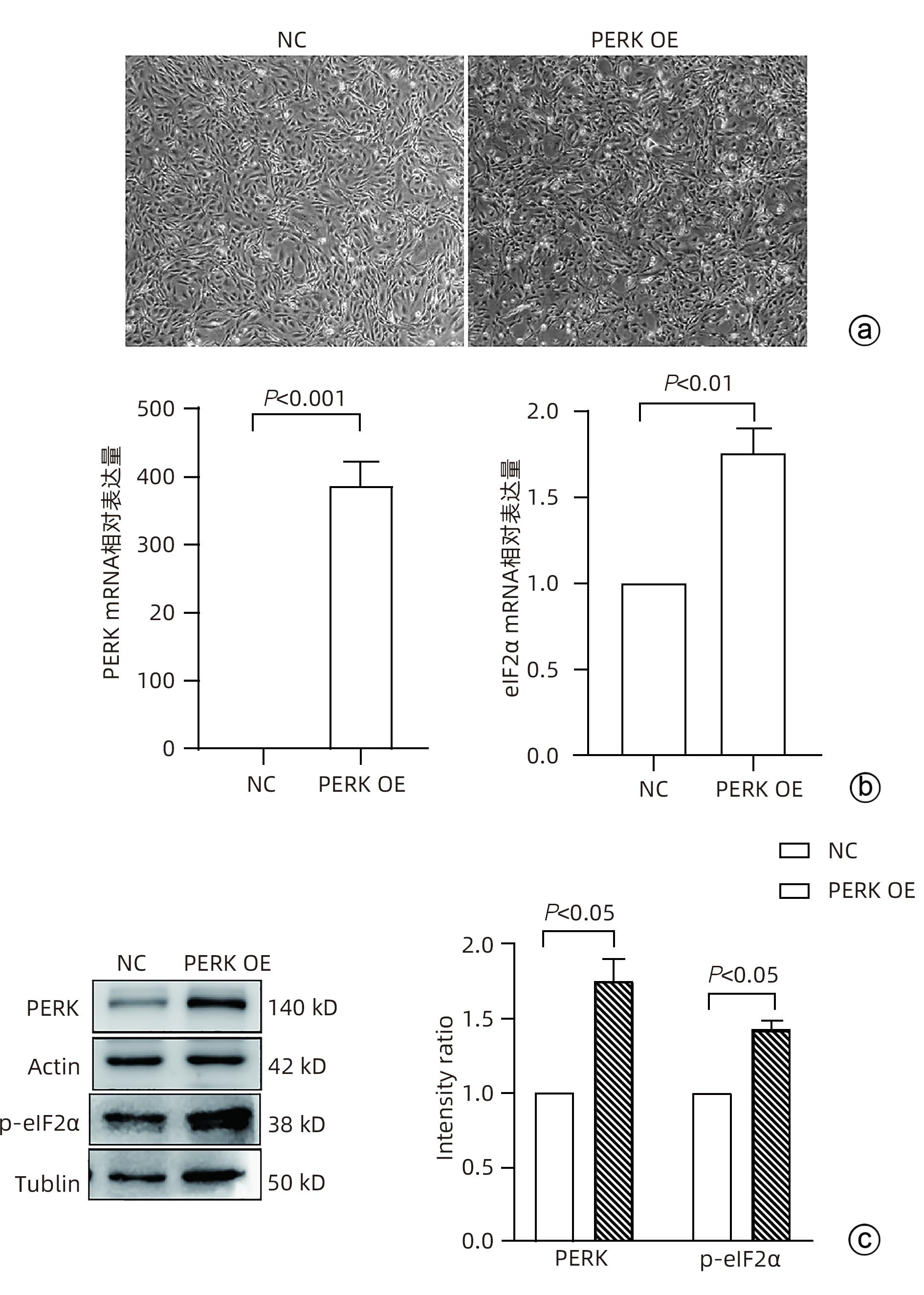
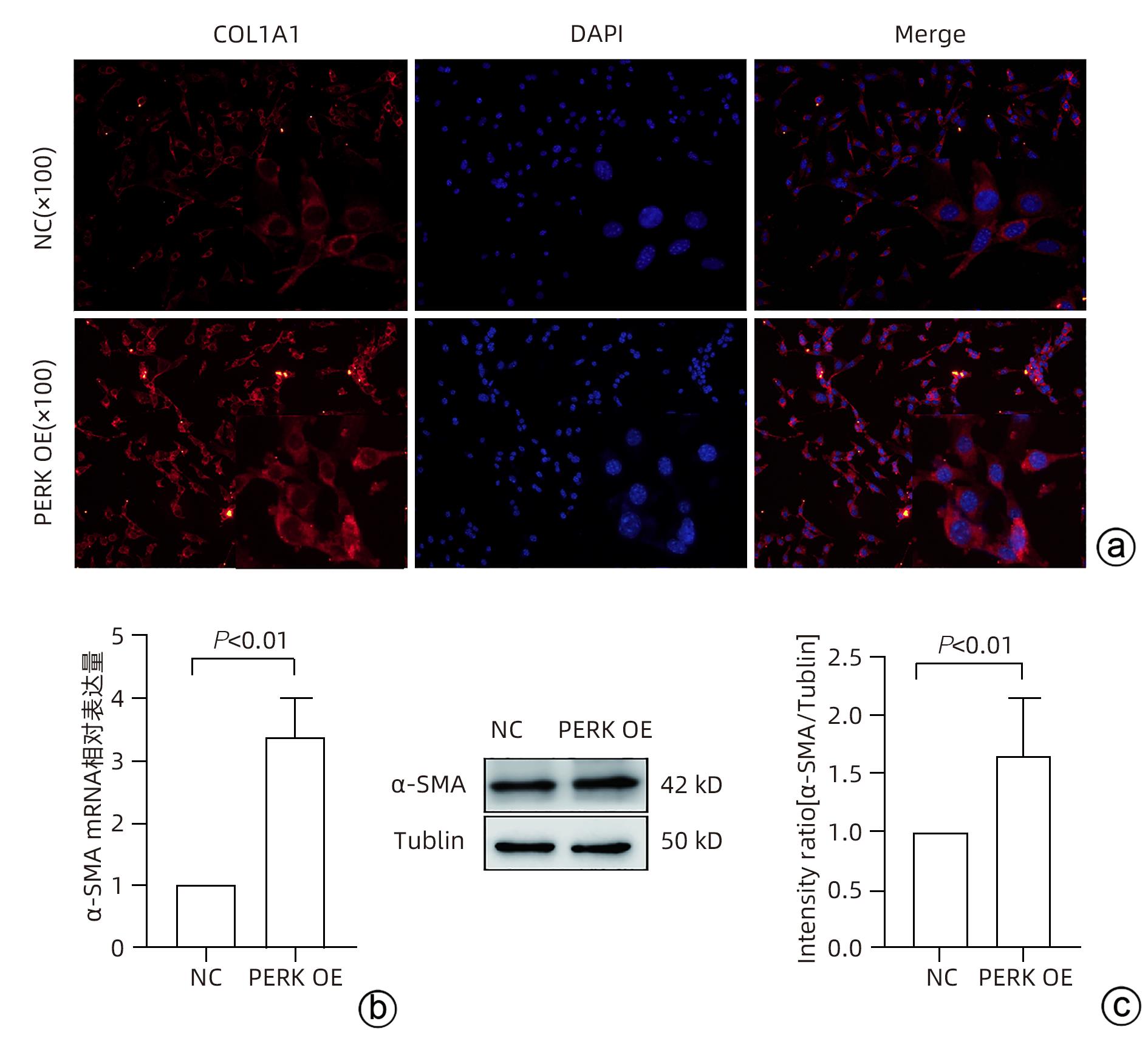
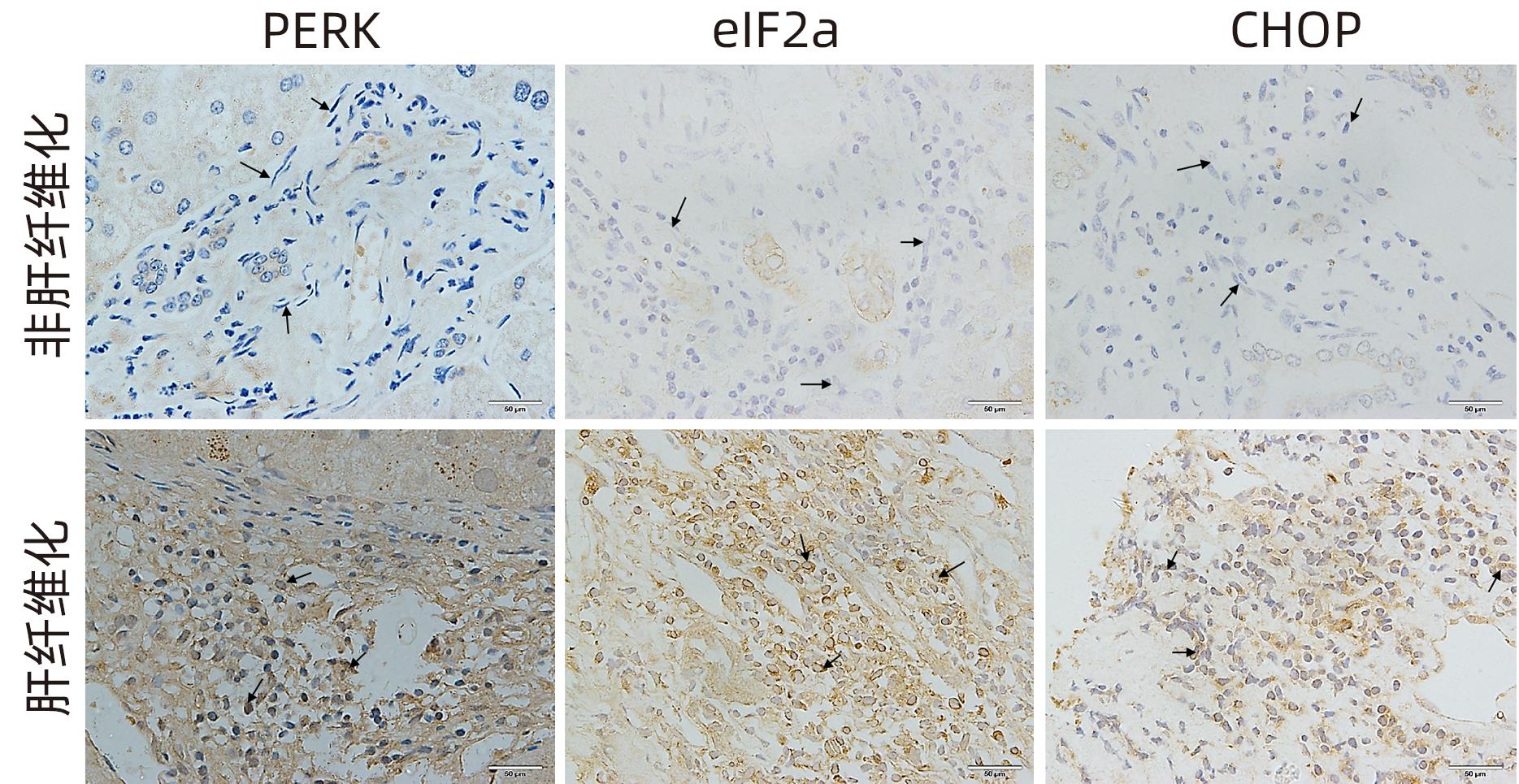
目的 探讨内质网应激蛋白激酶RNA样ER激酶(PERK)/真核生物翻译起始因子2α(eIF2α)信号通路对肝星状细胞(HSC)活化的影响。 方法 收集11例肝穿刺病理提示S1~S4肝纤维化患者和9例肝血管瘤、肝腺瘤患者术后周围正常肝组织病理切片,进一步行组织免疫组化检测PERK、eIF2α、C/EBP环磷酸腺苷反应元件结合转录因子同源蛋白(CHOP)表达情况;使用不同浓度的内质网应激诱导剂毒胡萝卜素(0、125、250、500、1 000 nmol/L)作用于人HSC-LX2,使用qRT-PCR检测PERK mRNA及Western Blot检测PERK、肌醇需要酶1(IRE1)、激活转录因子6(ATF6)、CHOP及α平滑肌肌动蛋白(α-SMA)表达水平。使用慢病毒转染构建PERK稳定过表达LX-2组及对照组,并通过qRT-PCR检测PERK、eIF2α、α-SMA mRNA,Western Blot检测PERK、p-eIF2α、α-SMA蛋白表达,免疫荧光检测胶Ⅰ型原蛋白(COL1A1)表达。符合正态分布的计量资料两组间比较采用成组t检验,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;不符合正态分布的计数资料,两组间比较采用Mann-Whitney U秩和检验。 结果 与正常肝组织相比,肝纤维化患者肝组织中PERK、eIF2α及CHOP表达升高,差异均有统计学意义(Z=-3.56、t=-5.75、Z=-3.52,P值均<0.001)。不同浓度毒胡萝卜素作用后,与溶媒组相比,内质网相关蛋白PERK、CHOP、IRE1、ATF6及α-SMA蛋白表达显著升高(P值均<0.05)。与对照空载慢病毒组相比,PERK稳定过表达组PERK mRNA、eIF2α mRNA、α-SMA mRNA表达及PERK、p-eIF2α、α-SMA蛋白表达明显升高(P值均<0.05)。细胞免疫荧光结果提示,PERK过表达组COL1A1表达升高(P<0.05)。 结论 PERK过表达可诱导LX-2细胞α-SMA、胶原蛋白COL1A1表达,提示PERK信号通路可能是HSC活化的重要机制之一。 Abstract:Objective To investigate the effect of the protein kinase RNA-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2α (eIF2α) pathway in endoplasmic reticulum stress on the activation of hepatic stellate cells (HSC). Methods Pathological sections of normal liver tissue after surgery were collected from 11 patients with hepatic fibrosis (S1-S4) and 9 patients with hepatic hemangioma and hepatic adenoma confirmed by liver biopsy, and immunohistochemistry was used to measure the protein expression levels of PERK, eIF2α, and C/EBP homologous protein (CHOP). Human HSC-LX2 cells were treated with different concentrations of the endoplasmic reticulum stress inducer thapsigargin (0, 125, 250, 500, and 1 000 nmol/L), and qRT-PCR was used to measure the mRNA expression level of PERK, while Western blot was used to measure the protein expression levels of PERK, inositol requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), CHOP, and α-smooth muscle actin (α-SMA). The method of lentivirus transfection was used to construct a PERK stable overexpression LX-2 group and a control group; qRT-PCR was used to measure the mRNA expression levels of PERK, eIF2α, and α-SMA, Western blot was used to measure the protein expression levels of PERK, phosphorylated eIF2α (p-eIF2α), and α-SMA, and immunofluorescence assay was used to measure the expression of collagen type I alpha 1 (COL1A1). The independent samples t-test was used for comparison of normally distributed continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups. Results Compared with normal liver tissue, the liver tissue of patients with hepatic fibrosis had significantly higher expression levels of PERK, eIF2α, and CHOP (Z=-3.56, t=-5.75, Z=-3.52, all P<0.001). Compared with the solvent group, the groups treated with different concentrations of thapsigargin had significant increases in the expression levels of the endoplasmic reticulum-associated proteins PERK, CHOP, IRE1, ATF6, and α-SMA (all P<0.05). Compared with the control group, the PERK stable overexpression group had significant increases in the mRNA expression levels of PERK, eIF2α, and α-SMA and the protein expression levels of PERK, p-eIF2α, and α-SMA (all P<0.05), and immunofluorescence assay showed a significant increase in the expression level of COL1A1 in the PERK stable overexpression group (P<0.05). Conclusion PERK overexpression can induce the expression of α-SMA and COL1A1 in LX-2 cells, suggesting that the PERK signaling pathway might be one of the important mechanisms of HSC activation. -
表 1 引物序列
Table 1. Primer sequences
引物 序列 PERK R: 5′-CATTGGGGTCAGAACCGT-3′ F: 5′-ATCGCAGAGGCAGTGGAG-3′ eIF2α R: 5′-TCACACGTAGTAGCAAAAGAACC-3′ F: 5′-GCCGCTAAACTTGCATATCTTCA-3′ α-SMA R: 5′-GCAGGGTGGGATGCTCTT-3′ F: 5′-GGGTGATGGTGGGAATGG-3′ β-actin R: 5′-CCACATAGGAATCCTTCTGACC-3′ F: 5′-CTTCGCGGGCGACGAT-3′ 表 2 肝纤维化患者与非肝纤维化患者病理切片细胞着色比例及染色强度得分
Table 2. The final score of cell staining proportion and staining intensity in hepatic fibrosis and non-hepatic fibrosis pathological sections
指标 非肝纤维化 患者(n=9) 肝纤维化 患者(n=11) 统计值 P值 PERK 0.50(0.10~1.13) 6.10(4.30~7.73) Z=-3.56 <0.001 eIF2α 1.46±0.89 4.83±1.52 t=-5.75 <0.001 CHOP 0.50(0.00~0.75) 3.00(2.00~4.75) Z=-3.52 <0.001 -
[1] HE YH, YAN YJ, ZHANG SF. Quantitative liver surface nodularity score based on imaging for assessment of early cirrhosis in patients with chronic liver disease: A protocol for systematic review and meta-analysis[J]. Medicine, 2021, 100( 4): e23636. DOI: 10.1097/MD.0000000000023636. [2] GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet Gastroenterol Hepatol, 2020, 5( 3): 245- 266. DOI: 10.1016/S2468-1253(19)30349-8. [3] ZHANG J, GUO JF, YANG NN, et al. Endoplasmic reticulum stress-mediated cell death in liver injury[J]. Cell Death Dis, 2022, 13( 12): 1051. DOI: 10.1038/s41419-022-05444-x. [4] XIA SW, WANG ZM, SUN SM, et al. Endoplasmic reticulum stress and protein degradation in chronic liver disease[J]. Pharmacol Res, 2020, 161: 105218. DOI: 10.1016/j.phrs.2020.105218. [5] SHAN S, ZHAO LH, MA H, et al. Definition, etiology, and epidemiology of liver cirrhosis[J]. J Clin Hepatol, 2021, 37( 1): 14- 16. DOI: 10.3969/j.issn.1001-5256.2021.01.003.单姗, 赵连晖, 马红, 等. 肝硬化的定义、病因及流行病学[J]. 临床肝胆病杂志, 2021, 37( 1): 14- 16. DOI: 10.3969/j.issn.1001-5256.2021.01.003. [6] XU LY, WEI ST, DONG Y, et al. Regulatory effect of lncRNA MALAT1 on activation of hepatic stellate cell and its mechanism[J]. J Jilin Univ Med Ed, 2023, 49( 3): 697- 705. DOI: 10.13481/j.1671-587X.20230319.徐菱遥, 魏书堂, 董勇, 等. lncRNA MALAT1对肝星状细胞活化的调节作用及其机制[J]. 吉林大学学报(医学版), 2023, 49( 3): 697- 705. DOI: 10.13481/j.1671-587X.20230319. [7] TSUCHIDA T, FRIEDMAN SL. Mechanisms of hepatic stellate cell activation[J]. Nat Rev Gastroenterol Hepatol, 2017, 14( 7): 397- 411. DOI: 10.1038/nrgastro.2017.38. [8] SUN DL, GUO JB, WANG DD, et al. Effect of exosomes derived from mesenchymal stem cells on the proliferation and activation of hepatic stellate cells in vitro[J]. J Pract Hepatol, 2023, 26( 1): 11- 14. DOI: 10.3969/j.issn.1672-5069.2023.01.004.孙东磊, 郭金波, 王丹丹, 等. 间充质干细胞外泌体对体外肝星状细胞增殖和活化的影响[J]. 实用肝脏病杂志, 2023, 26( 1): 11- 14. DOI: 10.3969/j.issn.1672-5069.2023.01.004. [9] FABRE B, LIVNEH I, ZIV T, et al. Identification of proteins regulated by the proteasome following induction of endoplasmic reticulum stress[J]. Biochem Biophys Res Commun, 2019, 517( 2): 188- 192. DOI: 10.1016/j.bbrc.2019.07.040. [10] PETERKOVÁ L, KMONÍČKOVÁ E, RUML T, et al. Sarco/endoplasmic reticulum calcium ATPase inhibitors: Beyond anticancer perspective[J]. J Med Chem, 2020, 63( 5): 1937- 1963. DOI: 10.1021/acs.jmedchem.9b01509. [11] IBRAHIM IM, ABDELMALEK DH, ELFIKY AA. GRP78: A cell's response to stress[J]. Life Sci, 2019, 226: 156- 163. DOI: 10.1016/j.lfs.2019.04.022. [12] KOO JH, LEE HJ, KIM W, et al. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2[J]. Gastroenterology, 2016, 150( 1): 181- 193. e 8. DOI: 10.1053/j.gastro.2015.09.039. [13] MAIERS JL, MALHI H. Endoplasmic reticulum stress in metabolic liver diseases and hepatic fibrosis[J]. Semin Liver Dis, 2019, 39( 2): 235- 248. DOI: 10.1055/s-0039-1681032. [14] LIAO X, ZHAN W, LI R, et al. Irisin ameliorates endoplasmic reticulum stress and liver fibrosis through inhibiting PERK-mediated destabilization of HNRNPA1 in hepatic stellate cells[J]. Biol Chem, 2021, 402( 6): 703- 715. DOI: 10.1515/hsz-2020-0251. -



 PDF下载 ( 2128 KB)
PDF下载 ( 2128 KB)

 下载:
下载: