程序性死亡受体1(PD-1)单抗联合索拉非尼或仑伐替尼治疗肝功能Child-Pugh B级不可切除肝癌患者的效果分析
DOI: 10.12449/JCH240517
Efficacy and safety of anti-PD-1 monoclonal antibody combined with sorafenib or lenvatinib in treatment of patients with Child-Pugh class B unresectable hepatocellular carcinoma
-
摘要:
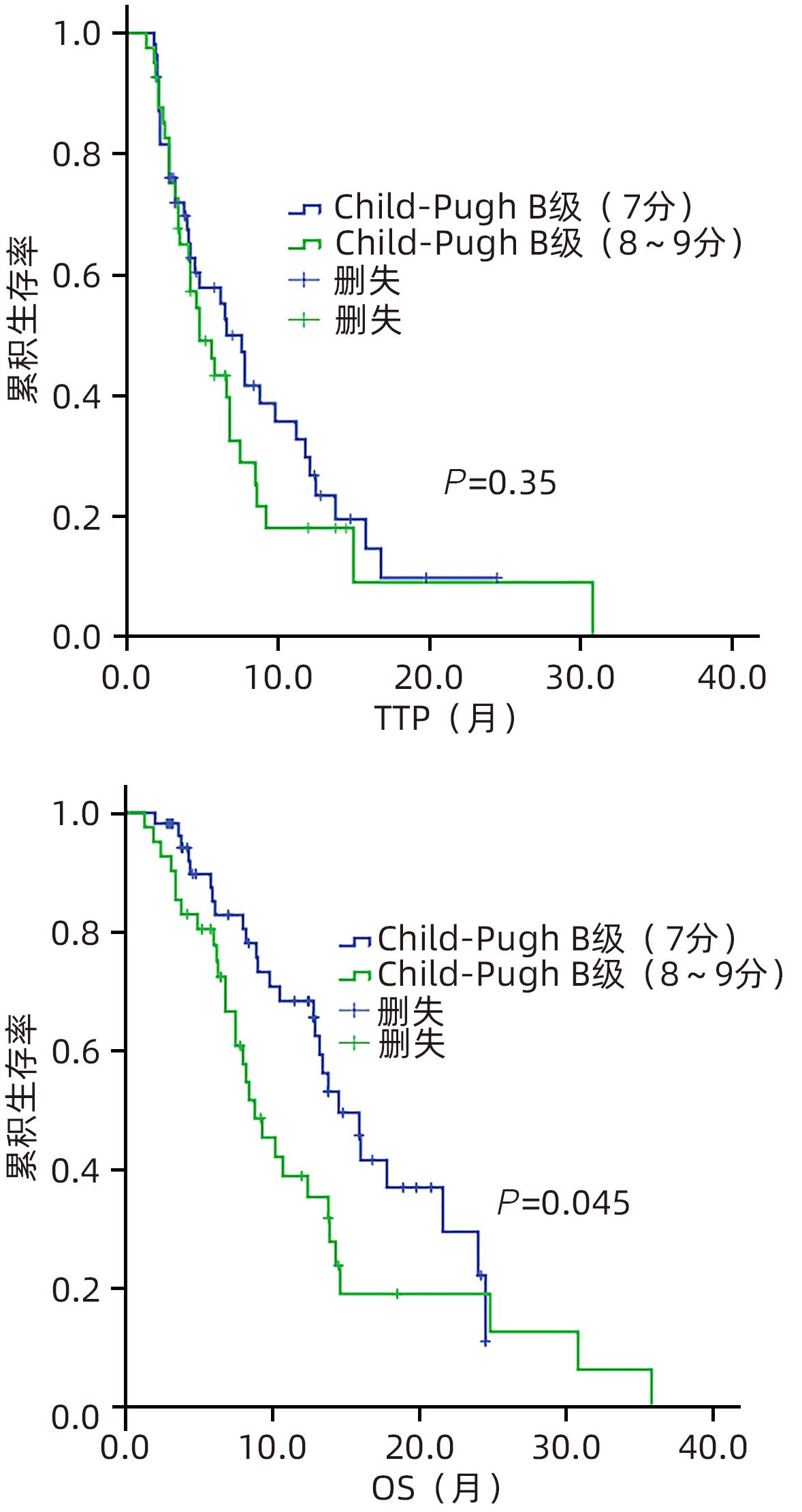
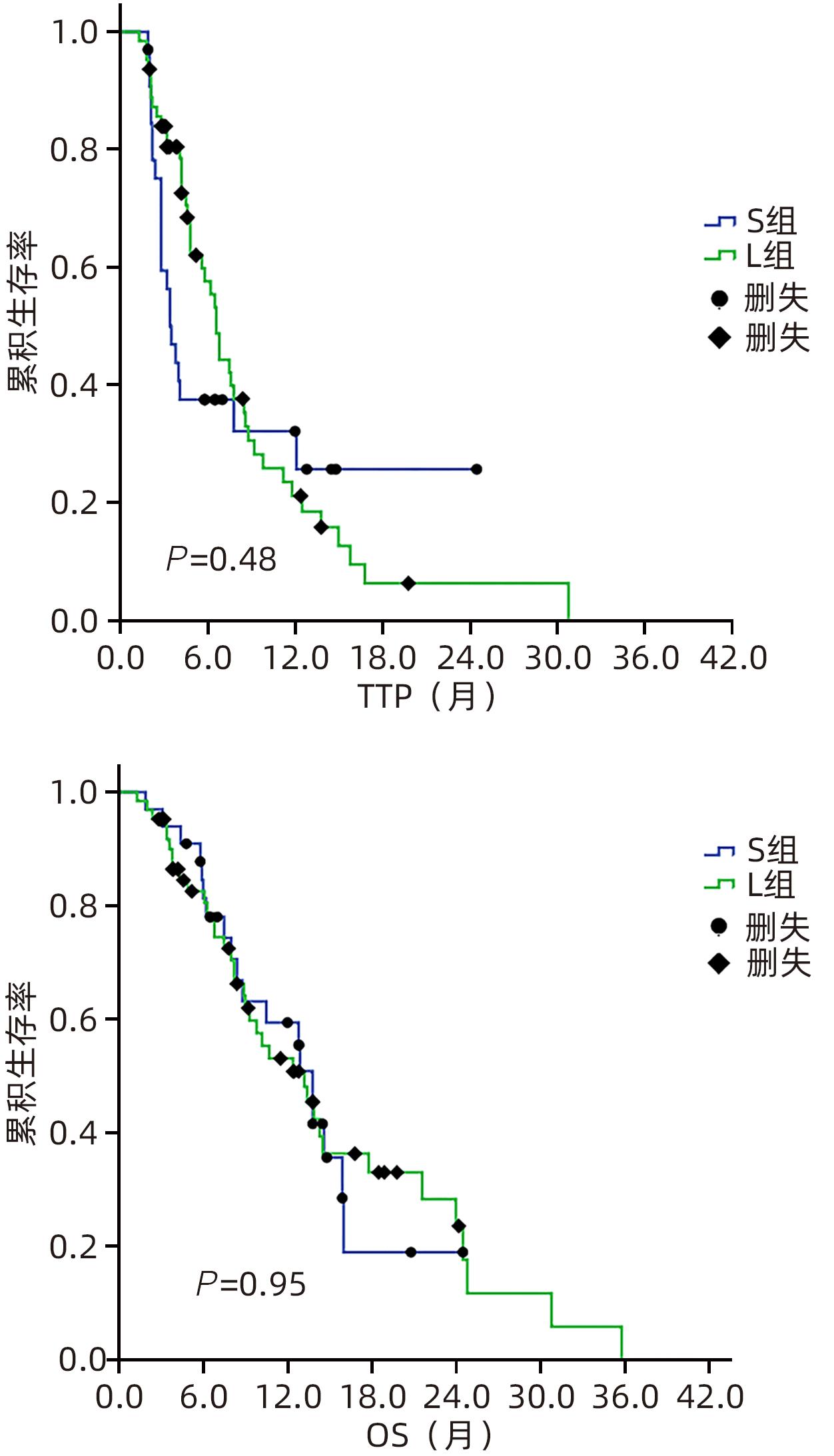
目的 回顾性分析酪氨酸激酶抑制剂联合免疫检查点抑制剂在肝功能Child-Pugh B级不可切除肝癌(uHCC)患者中的疗效和安全性。 方法 纳入2020年12月31日—2023年3月30日首都医科大学附属北京地坛医院收治的肝功能Child-Pugh B级的uHCC患者96例,接受仑伐替尼联合程序性细胞死亡-1(PD-1)抑制剂治疗者为L组(63例),接受索拉非尼联合PD-1抑制剂治疗者为S组(33例)。主要终点为客观缓解率(ORR),次要终点包括疾病进展时间(TTP)、总生存期(OS)、毒性、停药率和剂量调整率。符合正态分布的计量资料2组间比较采用成组t检验;非正态分布2组间比较采用Mann-Whitney U检验。计数资料2组间比较采用χ2检验。绘制生存曲线,运用Kaplan-Meier法计算2组患者的生存率,并利用Log-rank检验比较2组差异。通过Cox回归模型计算风险比(HR)和95%置信区间(95%CI),实现预后影响因素的多因素分析。 结果 96例uHCC患者中,Child-Pugh B级(7分)55例(57.3%),B级(8~9分)41例(42.7%)。L组患者的ORR显著高于S组(46.0% vs 15.2%,P=0.003)。L组和S组中位TTP(6.6个月 vs 3.5个月,P=0.48)或OS(13.8个月 vs 13.2个月,P=0.95)差异无统计学意义。Child-Pugh B级(7分)患者与Child-Pugh B级(8~9分)患者的中位TTP差异无统计学意义(6.6个月 vs 4.8个月,P=0.35),OS具有统计学意义(14.5个月 vs 8.8个月,P=0.045)。多因素分析显示,ORR是TTP(HR=0.18,95%CI:0.09~0.36,P<0.001)和OS(HR=0.20,95%CI:0.09~0.43,P<0.001)的保护因素。L组和S组总体不良反应(98.4% vs 97.0%)和≥3级不良反应的发生率(68.3% vs 63.6%)比较,差异均无统计学意义。L组和S组在剂量调整率(84.8% vs 70.2%)或停药率(56.1% vs 72.7%)方面无显著差异。 结论 与索拉非尼联合PD-1抑制剂方案相比,仑伐替尼联合PD-1抑制剂方案改善了Child-Pugh B级uHCC患者的ORR,但两组总体预后相似,总体安全性相当。 Abstract:Objective To investigate the safety and efficacy of tyrosine kinase inhibitors combined with immune checkpoint inhibitors in the treatment of patients with Child-Pugh class B unresectable hepatocellular carcinoma (uHCC). Methods A total of 96 patients with Child-Pugh class B uHCC who were admitted to Beijing Ditan Hospital, Capital Medical University, from December 31, 2020 to March 30, 2023 were enrolled as subjects, among whom 63 patients receiving lenvatinib combined with programmed death-1 (PD-1) inhibitor were enrolled as L group and 33 patients receiving sorafenib combined with PD-1inhibitor were enrolled as S group. The primary endpoint was objective response rate (ORR), and secondary endpoints included time to progression (TTP), overall survival (OS), toxicity, drug withdrawal rate, and dose adjustment rate. The The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Survival curves were plotted, and the Kaplan-Meier method was used to calculate the survival rate of patients in both groups, while the Log-rank test was used for comparison between the two groups. The Cox regression model was used to calculate hazard ratio (HR) and its 95% confidence interval (CI) and perform the multivariate analysis of influencing factors for prognosis. Results Among the 96 patients with uHCC, 55 (57.3%) had Child-Pugh class B (7 points) uHCC and 41 (42.7%) had Child-Pugh class B (8—9 points) uHCC. The L group had a significantly higher ORR than the S group (46.0% vs 15.2%, P=0.003), and there were no significant differences between the L group and the S group in median TTP (6.6 months vs 3.5 months, P=0.48) or OS (13.8 months vs 13.2 months, P=0.95). There was no significant difference in median TTP between the patients with Child-Pugh class B (7 points) uHCC and those with Child-Pugh class B (8—9 points) uHCC (6.6 months vs 4.8 months, P=0.35), while there was a significant difference in OS between these two groups of patients (14.5 months vs 8.8 months, P=0.045). The multivariate analysis showed that ORR was a protective factor for both TTP (HR=0.18, 95%CI: 0.09 — 0.36, P<0.001) and OS (HR=0.20, 95%CI: 0.09 — 0.43, P<0.001). There were no significant differences between the L group and the S group in the overall incidence rate of adverse reactions (98.4% vs 97.0%) and the incidence rate of grade≥3 adverse reactions (68.3% vs 63.6%), and there were also no significant differences between the two groups in dose adjustment rate (84.8% vs 70.2%) and drug withdrawal rate (56.1% vs 72.7%). Conclusion Compared with the regimen of sorafenib combined with PD-1 inhibitor, the regimen of lenvatinib combined with PD-1 inhibitor can improve the ORR of patients with Child-Pugh class B uHCC, with similar prognosis and safety profile between the two groups. -
表 1 2组患者一般资料及肿瘤情况比较
Table 1. Baseline patient demographic and disease characteristics
指标 合计 L组(n=63) S组(n=33) 统计值 P 值 年龄(岁) 62(29~82) 62(30~82) 64(29~74) Z=-0.10 0.92 男/女(例) 84/12 55/8 29/4 χ2=0.01 0.94 ECOG评分[例(%)] χ2=0.86 0.40 0分 49(51.0) 30(47.6) 19(57.6) 1分 47(49.0) 33(52.4) 14(42.4) 嗜肝病毒感染[例(%)] χ2=3.13 0.21 HBV 86(89.6) 54(85.7) 32(97.0) HCV 7(7.3) 6(9.5) 1(3.0) 非HBV或HCV 3(3.1) 3(4.8) 0(0.0) Child-Pugh评分 χ2=0.00 >0.05 B级(7分) 55(57.3) 36(57.1) 19(57.6) B级(8~9分) 41(42.7) 27(42.9) 14(42.4) 肝外转移[例(%)] χ2=0.58 0.52 有 40(41.7) 28(44.4) 12(36.4) 无 56(58.3) 35(55.6) 21(63.6) 合并门静脉癌栓[例(%)] χ2=0.32 0.66 有 59(61.5) 40(63.5) 19(57.6) 无 37(38.5) 23(36.5) 14(42.4) 靶病灶直径(cm) 8.9(8.8~10.8) 9.0(7.5~11.2) 7.9(6.2~10.2) Z=-1.61 0.07 肝内肿瘤个数[例(%)] χ2=0.56 0.50 1~3个 63(65.6) 43(68.3) 20(60.6) ≥4个 33(34.4) 20(31.7) 13(39.4) AFP[例(%)] χ2=0.83 0.39 ≥400 ng/mL 55(57.3) 34(54.0) 21(63.6) <400 ng/mL 41(42.7) 29(46.0) 12(36.4) 表 2 依据mRECIST标准评估的肿瘤反应率
Table 2. Response rates according to the mRECIST
疗效 L组(n=63) S组(n=33) χ2值 P值 CR[例(%)] 0(0.0) 0(0.0) PR[例(%)] 29(46.0) 5(15.2) 9.03 0.003 SD[例(%)] 25(39.7) 19(57.6) 2.79 0.134 PD[例(%)] 9(14.3) 9(27.3) 2.40 0.172 DCR(%) 85.7 72.7 2.40 0.171 表 3 依据iRECIST标准评估的肿瘤反应率
Table 3. Response rates according to the iRECIST
疗效 L组(n=63) S组(n=33) χ2值 P值 iCR[例(%)] 0(0.0) 0(0.0) iPR[例(%)] 29(46.0) 5(15.2) 9.03 0.003 iSD[例(%)] 25(39.7) 19(57.6) 2.79 0.134 iUPD[例(%)] 9(14.3) 9(27.3) 2.40 0.172 iCPD[例(%)] 9(14.3) 9(27.3) 2.40 0.172 DCR(%) 85.7 72.7 2.40 0.171 表 4 TTP的多变量Cox比例风险模型分析
Table 4. Multivariable Cox proportional hazards model for time to progression
变量 HR 95%CI P值 ORR(是=0/否=1) 0.18 0.09~0.36 <0.001 肿瘤最大径(≤7 cm=0/>7 cm=1) 0.55 0.29~1.06 0.074 AFP(≥400 ng/mL=0/<400 ng/mL=1) 1.00 0.58~1.72 >0.05 肝外转移(有=0/无=1) 1.44 0.86~2.42 0.171 表 5 OS的多变量Cox比例风险模型分析
Table 5. Multivariable Cox proportional hazards model for overall survival
变量 HR 95%CI P值 ORR(是=0/否=1) 0.20 0.09~0.43 <0.001 肿瘤最大径(≤7 cm=0/>7 cm=1) 0.45 0.21~0.99 0.048 AFP(≥400 ng/mL=0/<400 ng/mL=1) 1.06 0.57~1.97 0.874 Child-Pugh分级(7分=0/8~9分=1) 1.29 0.72~2.31 0.403 -
[1] MITTAL S, EL-SERAG HB, SADA YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2016, 14( 1): 124- 131. e 1. DOI: 10.1016/j.cgh.2015.07.019. [2] CHAPIN WJ, HWANG WT, KARASIC TB, et al. Comparison of nivolumab and sorafenib for first systemic therapy in patients with hepatocellular carcinoma and Child-Pugh B cirrhosis[J]. Cancer Med, 2023, 12( 1): 189- 199. DOI: 10.1002/cam4.4906. [3] MARRERO JA, KUDO M, VENOOK AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study[J]. J Hepatol, 2016, 65( 6): 1140- 1147. DOI: 10.1016/j.jhep.2016.07.020. [4] MCNAMARA MG, SLAGTER AE, NUTTALL C, et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis[J]. Eur J Cancer, 2018, 105: 1- 9. DOI: 10.1016/j.ejca.2018.09.031. [5] CHOI WM, LEE DB, SHIM JH, et al. Effectiveness and safety of nivolumab in child-pugh B patients with hepatocellular carcinoma: A real-world cohort study[J]. Cancers(Basel), 2020, 12( 7): 1968. DOI: 10.3390/cancers12071968. [6] OGUSHI K, CHUMA M, UOJIMA H, et al. Safety and efficacy of lenvatinib treatment in child-pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: A multicenter analysis[J]. Clin Exp Gastroenterol, 2020, 13: 385- 396. DOI: 10.2147/CEG.S256691. [7] KUDO M, MATILLA A, SANTORO A, et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis[J]. J Hepatol. 2021, 75( 3): 600- 609. DOI: 10.1016/j.jhep.2021.04.047. [8] FINN RS, QIN SK, IKEDA M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma[J]. N Engl J Med, 2020, 382( 20): 1894- 1905. DOI: 10.1056/NEJMoa1915745. [9] KUDO M, FINN RS, QIN SK, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391( 10126): 1163- 1173. DOI: 10.1016/S0140-6736(18)30207-1. [10] FAN J, GAO Q. Immunotherapy for hepacellular carcinoma: where there is hope, there is brightness[J]. Chin J Dig Surg, 2022, 21( 2): 199- 204. DOI: 10.3760/cma.j.cn115610-20220215-00080.樊嘉, 高强. 肝癌的免疫治疗:有希望便是光明[J]. 中华消化外科杂志, 2022, 21( 2): 199- 204. DOI: 10.3760/cma.j.cn115610-20220215-00080. [11] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [12] MISCHEL AM, ROSIELLE DA. Eastern cooperative oncology group performance status#434[J]. J Palliat Med, 2022, 25( 3): 508- 510. DOI: 10.1089/jpm.2021.0599. [13] LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72( 2): 288- 306. DOI: 10.1016/j.jhep.2019.09.026. [14] SEYMOUR L, BOGAERTS J, PERRONE A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics[J]. Lancet Oncol, 2017, 18( 3): e143- e152. DOI: 10.1016/s1470-2045(17)30074-8. [15] US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events(CTCAE), Version 5.0. 2017. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0 https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0 [16] ABOU-ALFA GK, AMADORI D, SANTORO A, et al. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma(HCC) and child-pugh A versus B cirrhosis[J]. Gastrointest Cancer Res, 2011, 4( 2): 40- 44. [17] KØSTNER AH, SORENSEN M, OLESEN RK, et al. Sorafenib in advanced hepatocellular carcinoma: A nationwide retrospective study of efficacy and tolerability[J]. Sci World J, 2013, 2013: 931972. DOI: 10.1155/2013/931972. [18] XIE ER, YEO YH, SCHEINER B, et al. Immune checkpoint inhibitors for child-pugh class B advanced hepatocellular carcinoma: A systematic review and meta-analysis[J]. JAMA Oncol, 2023, 9( 10): 1423- 1431. DOI: 10.1001/jamaoncol.2023.3284. [19] FINN RS, KUDO M, MERLE P, et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line(1L) therapy for advanced hepatocellular carcinoma(aHCC)[J]. Ann Oncol, 2022, 33: S1401. DOI: 10.1016/j.annonc.2022.08.031. [20] QIN SK, CHAN SL, GU SZ, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma(CARES-310): A randomised, open-label, international phase 3 study[J]. Lancet, 2023, 402( 10408): 1133- 1146. DOI: 10.1016/S0140-6736(23)00961-3. [21] REN ZG, XU JM, BAI YX, et al. Sintilimab plus a bevacizumab biosimilar(IBI305) versus sorafenib in unresectable hepatocellular carcinoma(ORIENT-32): A randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22( 7): 977- 990. DOI: 10.1016/S1470-2045(21)00252-7. [22] WU CJ, LEE PC, HUNG YW, et al. Lenvatinib plus pembrolizumab for systemic therapy-naïve and-experienced unresectable hepatocellular carcinoma[J]. Cancer Immunol Immunother, 2022, 71( 11): 2631- 2643. DOI: 10.1007/s00262-022-03185-6. [23] XU JM, SHEN J, GU SZ, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma(RESCUE): A nonrandomized, open-label, phase II trial[J]. Clin Cancer Res, 2021, 27( 4): 1003- 1011. DOI: 10.1158/1078-0432.CCR-20-2571. [24] TSUCHIYA K, KUROSAKI M, SAKAMOTO A, et al. The real-world data in Japanese patients with unresectable hepatocellular carcinoma treated with lenvatinib from a nationwide multicenter study[J]. Cancers(Basel), 2021, 13( 11): 2608. DOI: 10.3390/cancers13112608. [25] KUO HY, CHIANG NJ, CHUANG CH, et al. Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma[J]. Oncol Res Treat, 2020, 43( 5): 211- 220. DOI: 10.1159/000505933. [26] CASADEI GARDINI A, PUZZONI M, MONTAGNANI F, et al. Profile of lenvatinib in the treatment of hepatocellular carcinoma: Design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all Phase III trials[J]. Onco Targets Ther, 2019, 12: 2981- 2988. DOI: 10.2147/OTT.S192572. [27] PFISTER D, NÚÑEZ NG, PINYOL R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC[J]. Nature, 2021, 592( 7854): 450- 456. DOI: 10.1038/s41586-021-03362-0. [28] RIMINI M, RIMASSA L, UESHIMA K, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis[J]. ESMO Open, 2022, 7( 6): 100591. DOI: 10.1016/j.esmoop.2022.100591. -



 PDF下载 ( 874 KB)
PDF下载 ( 874 KB)

 下载:
下载: