-
摘要: 自身免疫性胰腺炎(AIP)是一种少见疾病,其诊断应基于对临床、影像学、血清学和病理学结果全面评估。目前将AIP分为两型:1型AIP被认为是与IgG4相关疾病的胰腺表现;2型AIP被视为与IgG4无关的胰腺特异性疾病。尽管1型和2型AIP的发病机制似乎不同,但二者的影像学表现相似,且均对类固醇激素有良好反应。本综述主要关注于AIP 2个亚型的病理组织学特征,尤其是内镜超声引导的细针穿刺活检组织的病理诊断难点,以提高AIP临床诊断的准确性。Abstract: Autoimmune pancreatitis (AIP) is a rare disease, and its diagnosis should be made based on a comprehensive evaluation of clinical, radiological, serological, and pathological findings. At present, AIP is classified into two subtypes of type 1 (identified as the pancreatic manifestation of IgG4-related disease) and type 2 (identified as the pancreas-specific disorder independent of IgG4). Although type 1 and type 2 AIP seem to have different pathogeneses, they tend to have similar radiological findings and exhibit a good response to corticosteroid therapy. This article mainly reviews the histopathological features of the two subtypes of AIP, especially the diagnostic challenges encountered in the interpretation of specimens obtained through endoscopic ultrasound-guided fine needle aspiration/biopsy, to as to help pathologists enhance the accuracy of the diagnosis of AIP.
-
Key words:
- Autoimmune Pancreatitis /
- Biopsy /
- Pathology /
- Diagnosis
-
表 1 1型AIP和2型AIP的临床特征对比
Table 1. Comparison of clinical features between type 1 AIP and type 2 AIP
项目 1型AIP 2型AIP 平均发病年龄 60岁(一般>40岁) 30岁(包括儿童) 男性占比(%) 80 50 血清IgG4水平升高(%) 80~90 10 其他器官累及(%) 50 0 合并发生炎症性肠病(%) <5 40 主要临床表现 腹痛/急性胰腺炎(%) 10 60 无痛性黄疸(%) 80 30 对激素的反应(%) ~100 ~100 复发(%) 30~50 <10 表 2 ICDC中1型和2型AIP的组织学诊断标准
Table 2. Histological diagnostic criteria for type 1 and type 2 AIP in ICDC
项目 诊断标准 1型AIP 1级 LPSP(空芯针活检/切除),至少符合以下3条:
(1)导管周围淋巴浆细胞浸润,无粒细胞;
(2)闭塞性静脉炎;
(3)席纹状纤维化;
(4)(>10个/HPF)IgG4阳性浆细胞
2级 LPSP(空芯针活检),符合以下任何2条:
(1)导管周围淋巴浆细胞浸润,无粒细胞浸润;
(2)闭塞性静脉炎;
(3)席纹状纤维化;
(4)丰富(>10个/HPF)IgG4阳性浆细胞
2型AIP 1级 IDCP(空芯针活检/切除),包括以下2条:
(1)中性粒细胞浸润导管壁伴或不伴腺泡内中性粒细胞浸润;
(2)IgG4阳性浆细胞缺乏或少量(0~10个/HPF)
2级 IDCP(空芯针活检/切除),包括以下2条:
(1)腺泡内中性粒细胞和淋巴浆细胞浸润;
(2)IgG4阳性浆细胞缺乏或少量(0~10个/HPF)
注:HPF,高倍镜。
表 3 ADM和高分化导管腺癌的鉴别
Table 3. Identification of ADM and well-differentiated ductal adenocarcinoma
项目 ADM PDAC 带间质的腺体分布 只位于小叶内 腺癌浸润,小叶结构消失或者腺癌浸润至小叶间质 腺体形状 管腔和腺体轮廓不清 低倍即可见明显的腺体 促结缔组织反应 无 有 游离细胞簇 数量 少量 多量 形状 疏松连接的细胞簇 明显的腺体和细胞栅栏排列 核形状 小、圆、规则 增大、染色质深,大小和形状不规则 表 4 1型AIP和2型AIP的组织学改变对比
Table 4. Comparison of histological changes between type 1 AIP and type 2 AIP
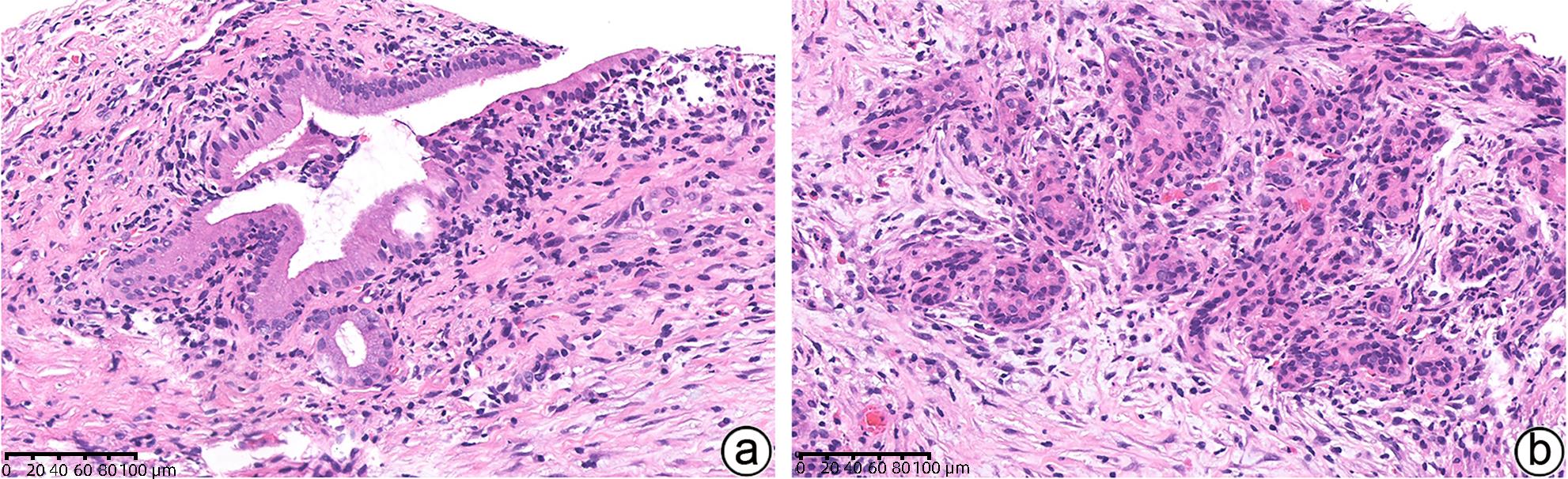
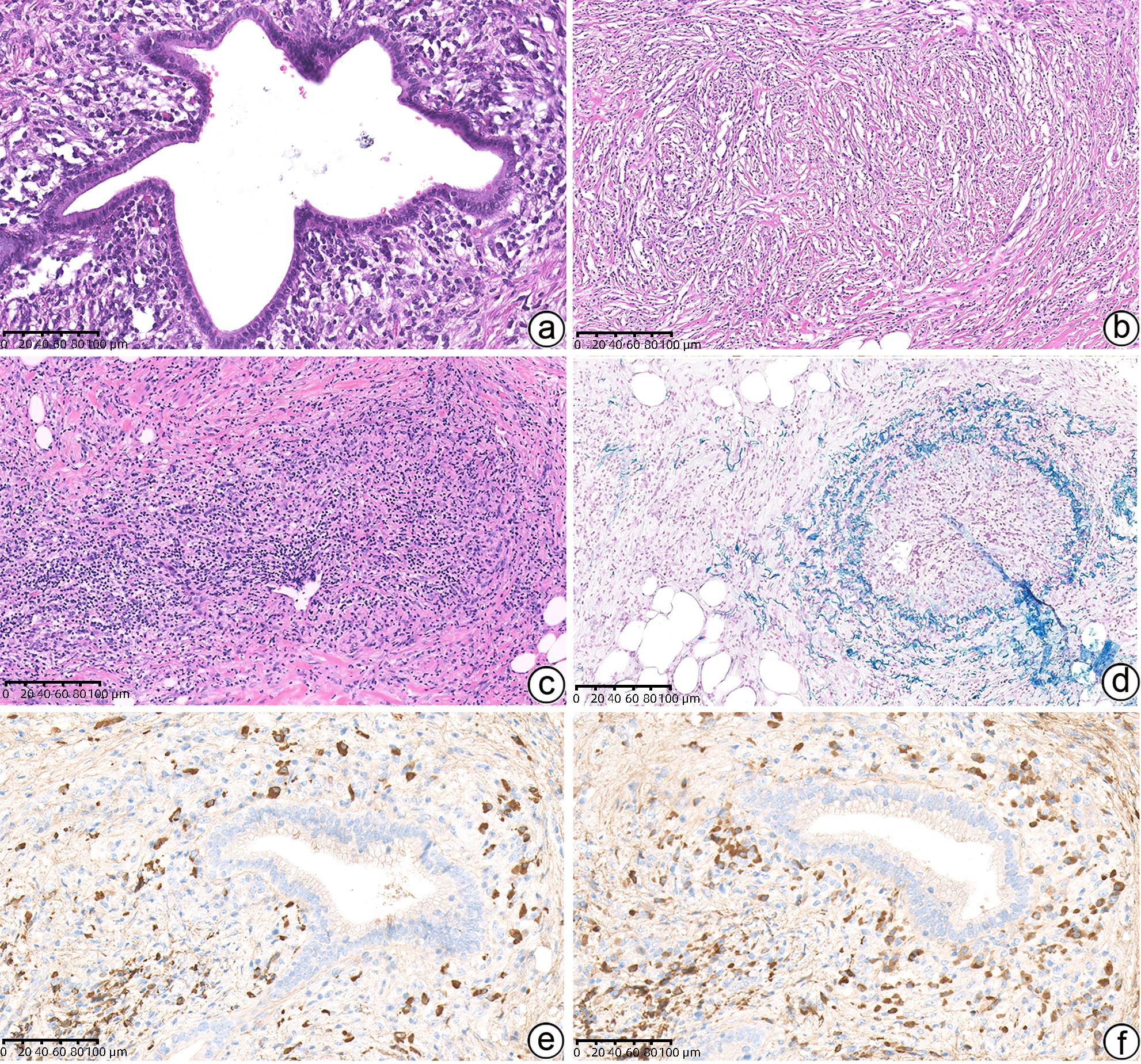
项目 1型AIP 2型AIP 炎症模式 纤维炎症累及胰管、小叶、静脉、胆总管 纤维炎症主要累及胰管以及胰腺内胆总管,小叶和静脉不明显 浸润 明显淋巴浆细胞浸润,常伴随嗜酸性粒细胞,少量中性粒细胞 多量淋巴浆细胞浸润,明显的中性粒细胞浸润中等及小导管、腺泡 胰管 致密性导管周围炎症,导管上皮保存完整 致密性导管周围炎症、中性粒细胞浸润毁损导管上皮
(粒细胞上皮病变)
胰管内蛋白栓和结石 无 无 小叶 淋巴浆细胞浸润,累及替换腺泡组织 淋巴浆细胞斑片状浸润,常混合中性粒细胞 静脉 闭塞性静脉炎 闭塞性静脉炎罕见 动脉 明显的动脉炎症累及罕见 无动脉炎症累及 胰腺周围脂肪组织 纤维炎症过程可能延伸至胰腺周围区域 炎症通常局限于胰腺内 IgG4免疫组化 丰富(>10个/HPF)IgG4+浆细胞 少量或无IgG4+浆细胞 -
[1] SHIMOSEGAWA T, CHARI ST, FRULLONI L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology[J]. Pancreas, 2011, 40( 3): 352- 358. DOI: 10.1097/MPA.0b013e3182142fd2. [2] DELLA-TORRE E, LANZILLOTTA M, DOGLIONI C. Immunology of IgG4-related disease[J]. Clin Exp Immunol, 2015, 181( 2): 191- 206. DOI: 10.1111/cei.12641. [3] SAH RP, CHARI ST, PANNALA R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis[J]. Gastroenterology, 2010, 139( 1): 140- 148; quize12- 3. DOI: 10.1053/j.gastro.2010.03.054. [4] HART PA, LEVY MJ, SMYRK TC, et al. Clinical profiles and outcomes in idiopathic duct-centric chronic pancreatitis(type 2 autoimmune pancreatitis): The Mayo Clinic experience[J]. Gut, 2016, 65( 10): 1702- 1709. DOI: 10.1136/gutjnl-2015-309275. [5] OH D, SONG TJ, MOON SH, et al. Erratum: Type 2 autoimmune pancreatitis(idiopathic duct-centric pancreatitis) highlighting patients presenting as clinical acute pancreatitis: A single-center experience[J]. Gut Liver, 2019, 13( 5): 583. DOI: 10.5009/gnl13051. [6] LORENZO D, MAIRE F, STEFANESCU C, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases[J]. Clin Gastroenterol Hepatol, 2018, 16( 1): 59- 67. DOI: 10.1016/j.cgh.2017.07.033. [7] ZEN Y. Type 2 autoimmune pancreatitis: Consensus and controversies[J]. Gut Liver, 2022, 16( 3): 357- 365. DOI: 10.5009/gnl210241. [8] OKAZAKI K, KAWA S, KAMISAWA T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: Revised proposal[J]. J Gastroenterol, 2006, 41( 7): 626- 631. DOI: 10.1007/s00535-006-1868-0. [9] CHARI ST, SMYRK TC, LEVY MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience[J]. Clin Gastroenterol Hepatol, 2006, 4( 8): 1010- 1016;quiz934. DOI: 10.1016/j.cgh.2006.05.017. [10] CHARI ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: Introducing the Mayo Clinic’s HISORt criteria[J]. J Gastroenterol, 2007, 42( Suppl 18): 39- 41. DOI: 10.1007/s00535-007-2046-8. [11] MARUYAMA M, WATANABE T, KANAI K, et al. International consensus diagnostic criteria for autoimmune pancreatitis and its Japanese amendment have improved diagnostic ability over existing criteria[J]. Gastroenterol Res Pract, 2013, 2013: 456965. DOI: 10.1155/2013/456965. [12] KHANDELWAL A, INOUE D, TAKAHASHI N. Autoimmune pancreatitis: An update[J]. Abdom Radiol, 2020, 45( 5): 1359- 1370. DOI: 10.1007/s00261-019-02275-x. [13] ZEN Y, NAKANUMA Y. IgG4-related disease: A cross-sectional study of 114 cases[J]. Am J Surg Pathol, 2010, 34( 12): 1812- 1819. DOI: 10.1097/PAS.0b013e3181f7266b. [14] ZHANG LZ, NOTOHARA K, LEVY MJ, et al. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis[J]. Mod Pathol, 2007, 20( 1): 23- 28. DOI: 10.1038/modpathol.3800689. [15] DESHPANDE V, ZEN Y, CHAN JK, et al. Consensus statement on the pathology of IgG4-related disease[J]. Mod Pathol, 2012, 25( 9): 1181- 1192. DOI: 10.1038/modpathol.2012.72. [16] SATO Y, KOJIMA M, TAKATA K, et al. Systemic IgG4-related lymphadenopathy: A clinical and pathologic comparison to multicentric Castleman’s disease[J]. Mod Pathol, 2009, 22( 4): 589- 599. DOI: 10.1038/modpathol.2009.17. [17] KITAGAWA S, ZEN Y, HARADA K, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis(Küttner’s tumor)[J]. Am J Surg Pathol, 2005, 29( 6): 783- 791. DOI: 10.1097/01.pas.0000164031.59940.fc. [18] SATO Y, KOJIMA M, TAKATA K, et al. Multicentric Castleman’s disease with abundant IgG4-positive cells: A clinical and pathological analysis of six cases[J]. J Clin Pathol, 2010, 63( 12): 1084- 1089. DOI: 10.1136/jcp.2010.082958. [19] ZEN Y. Autoimmune pancreatitis: Biopsy interpretation and differential diagnosis[J]. Semin Diagn Pathol, 2024, 41( 2): 79- 87. DOI: 10.1053/j.semdp.2024.01.001. [20] KAWAKAMI H, ZEN Y, KUWATANI M, et al. IgG4-related sclerosing cholangitis and autoimmune pancreatitis: Histological assessment of biopsies from Vater’s ampulla and the bile duct[J]. J Gastroenterol Hepatol, 2010, 25( 10): 1648- 1655. DOI: 10.1111/j.1440-1746.2010.06346.x. [21] CHHODA A, RUSTAGI T. EUS-guided needle biopsy for autoimmune pancreatitis[J]. Clin J Gastroenterol, 2020, 13( 5): 669- 677. DOI: 10.1007/s12328-020-01153-0. [22] YOON SB, MOON SH, SONG TJ, et al. Endoscopic ultrasound-guided fine needle aspiration versus biopsy for diagnosis of autoimmune pancreatitis: Systematic review and comparative meta-analysis[J]. Dig Endosc, 2021, 33( 7): 1024- 1033. DOI: 10.1111/den.13866. [23] FACCIORUSSO A, BARRESI L, CANNIZZARO R, et al. Diagnostic yield of endoscopic ultrasound-guided tissue acquisition in autoimmune pancreatitis: A systematic review and meta-analysis[J]. Endosc Int Open, 2021, 9( 1): E66- E75. DOI: 10.1055/a-1293-7279. [24] SHIMOSEGAWA T, Working Group Members of the Japan Pancreas Society, Health and Welfare of Japan Research Committee for Intractable Pancreatic Disease by the Ministry of Labor. The amendment of the Clinical Diagnostic Criteria in Japan(JPS2011) in response to the proposal of the International Consensus of Diagnostic Criteria(ICDC) for autoimmune pancreatitis[J]. Pancreas, 2012, 41( 8): 1341- 1342. DOI: 10.1097/MPA.0b013e3182706ed5. [25] KAWA S, KAMISAWA T, NOTOHARA K, et al. Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2018: Revision of Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2011[J]. Pancreas, 2020, 49( 1): e13- e14. DOI: 10.1097/MPA.0000000000001443. [26] NOTOHARA K. Biopsy diagnosis of type 1 autoimmune pancreatitis: Does it bring a conclusion or confusion?[J]. DEN Open, 2022, 2( 1): e82. DOI: 10.1002/deo2.82. [27] CAO L, WANG Y, WANG JL, et al. The role of EUS-guided fine needle aspiration in autoimmune pancreatitis: A single center prospective study[J]. Scand J Gastroenterol, 2018, 53( 12): 1604- 1610. DOI: 10.1080/00365521.2018.1534137. [28] KANNO A, MASAMUNE A, FUJISHIMA F, et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study[J]. Gastrointest Endosc, 2016, 84( 5): 797- 804. e 1. DOI: 10.1016/j.gie.2016.03.1511. [29] NOTOHARA K, KAMISAWA T, KANNO A, et al. Efficacy and limitations of the histological diagnosis of type 1 autoimmune pancreatitis with endoscopic ultrasound-guided fine needle biopsy with large tissue amounts[J]. Pancreatology, 2020, 20( 5): 834- 843. DOI: 10.1016/j.pan.2020.05.026. [30] NOTOHARA K, KAMISAWA T, FUKUSHIMA N, et al. Guidance for diagnosing autoimmune pancreatitis with biopsy tissues[J]. Pathol Int, 2020, 70( 10): 699- 711. DOI: 10.1111/pin.12994. [31] BASTURK O, HONG SM, WOOD LD, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas[J]. Am J Surg Pathol, 2015, 39( 12): 1730- 1741. DOI: 10.1097/PAS.0000000000000533. [32] DESHPANDE V, GUPTA R, SAINANI N, et al. Subclassification of autoimmune pancreatitis: A histologic classification with clinical significance[J]. Am J Surg Pathol, 2011, 35( 1): 26- 35. DOI: 10.1097/PAS.0b013e3182027717. [33] HAYASHI H, MIURA S, FUJISHIMA F, et al. Utility of endoscopic ultrasound-guided fine-needle aspiration and biopsy for histological diagnosis of type 2 autoimmune pancreatitis[J]. Diagnostics, 2022, 12( 10): 2464. DOI: 10.3390/diagnostics12102464. [34] CHARI ST, KLOEPPEL G, ZHANG LZ, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: The Honolulu consensus document[J]. Pancreatology, 2010, 10( 6): 664- 672. DOI: 10.1159/000318809. [35] SAYED AHMED A, ABREO M, THOMAS A, et al. Type 3 autoimmune pancreatitis(immune checkpoint inhibitor-induced pancreatitis)[J]. Curr Opin Gastroenterol, 2022, 38( 5): 516- 520. DOI: 10.1097/MOG.0000000000000873. -



 PDF下载 ( 1309 KB)
PDF下载 ( 1309 KB)

 下载:
下载: