奥沙利铂对肝星状细胞活化的影响及机制探讨
DOI: 10.12449/JCH240612
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:王存凯负责设计论文框架,起草论文;王存凯、王一军、谢肖立负责实验操作,研究过程的实施;王丹丹、白云负责数据整理、统计学分析;刘红玉负责论文修改;王玉珍、姜慧卿负责拟定写作思路,指导撰写文章并最后定稿。
Effect of oxaliplatin on the activation of hepatic stellate cells and its mechanism
-
摘要:
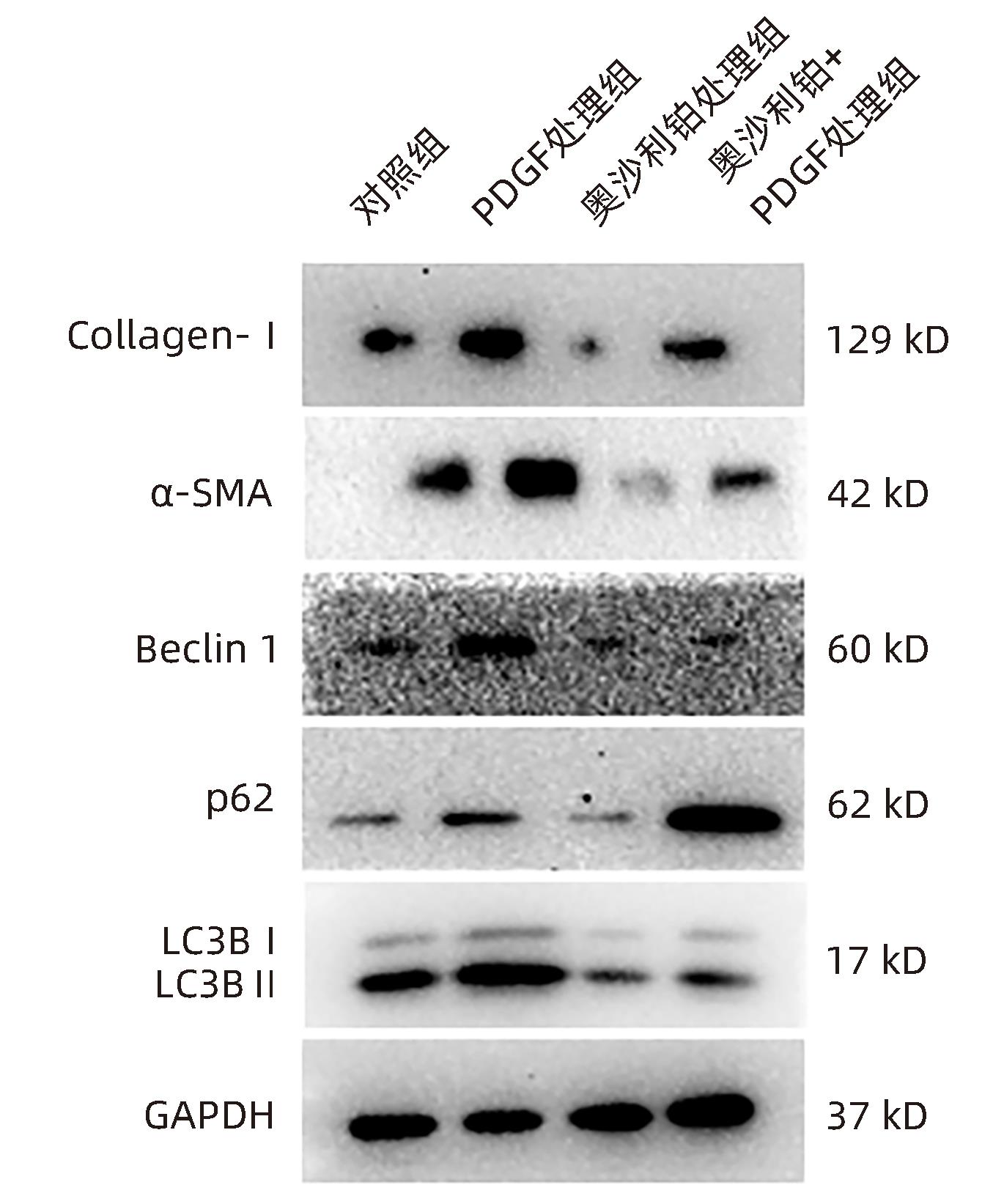
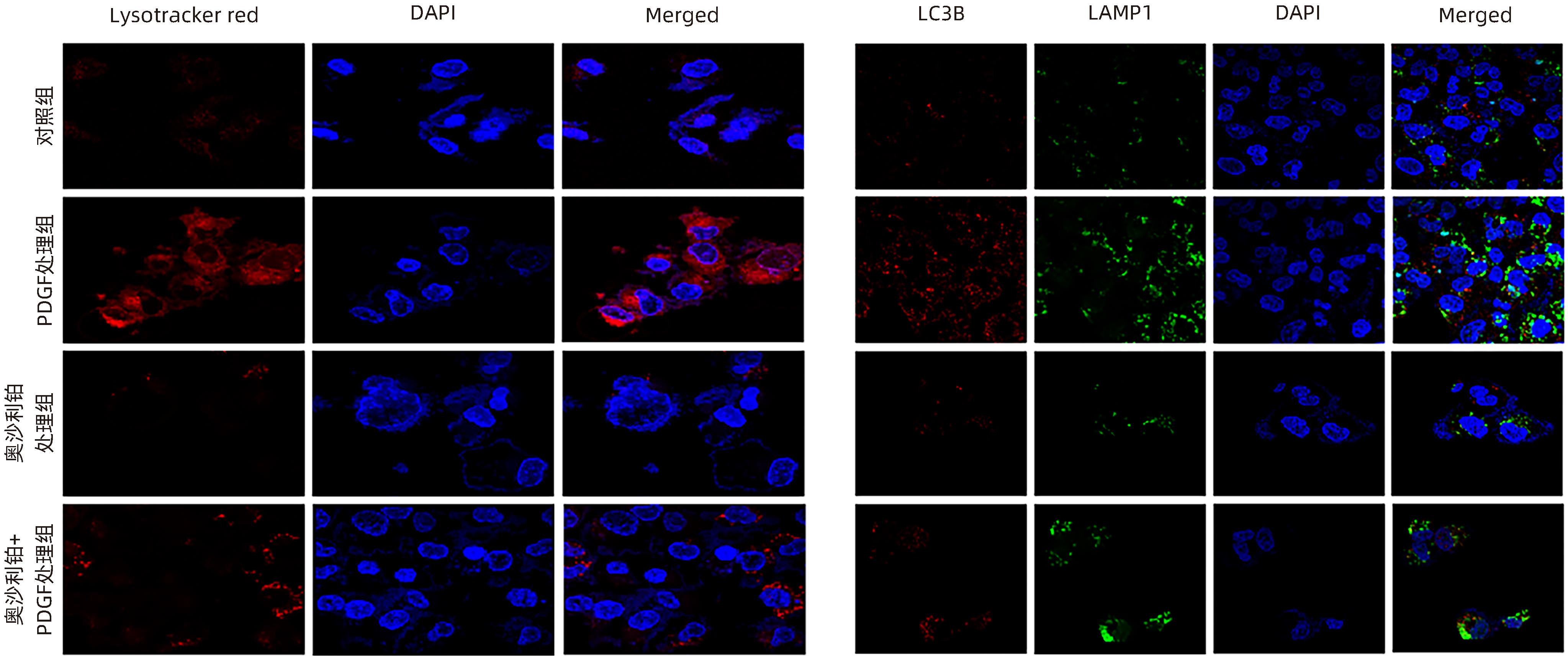
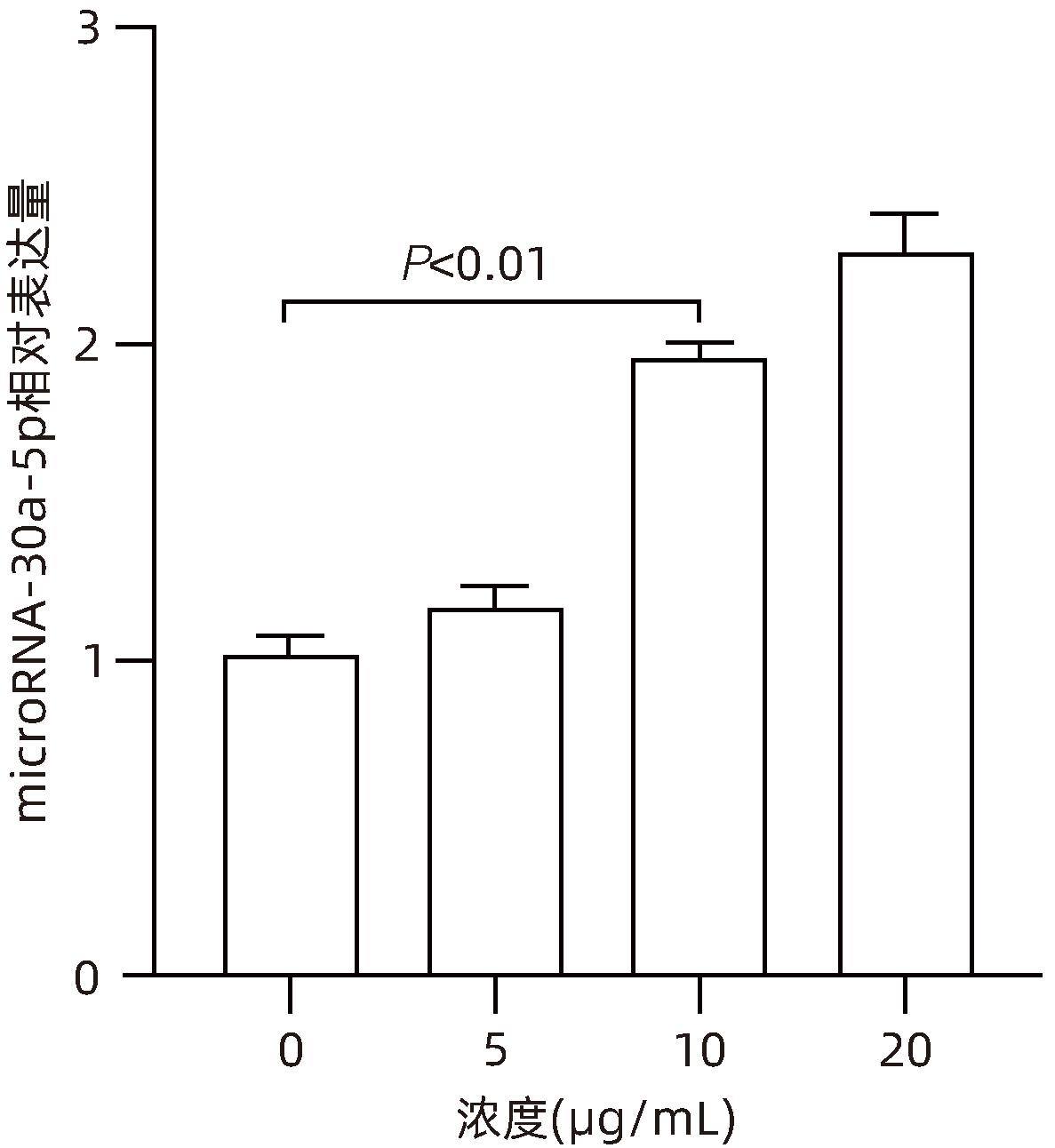
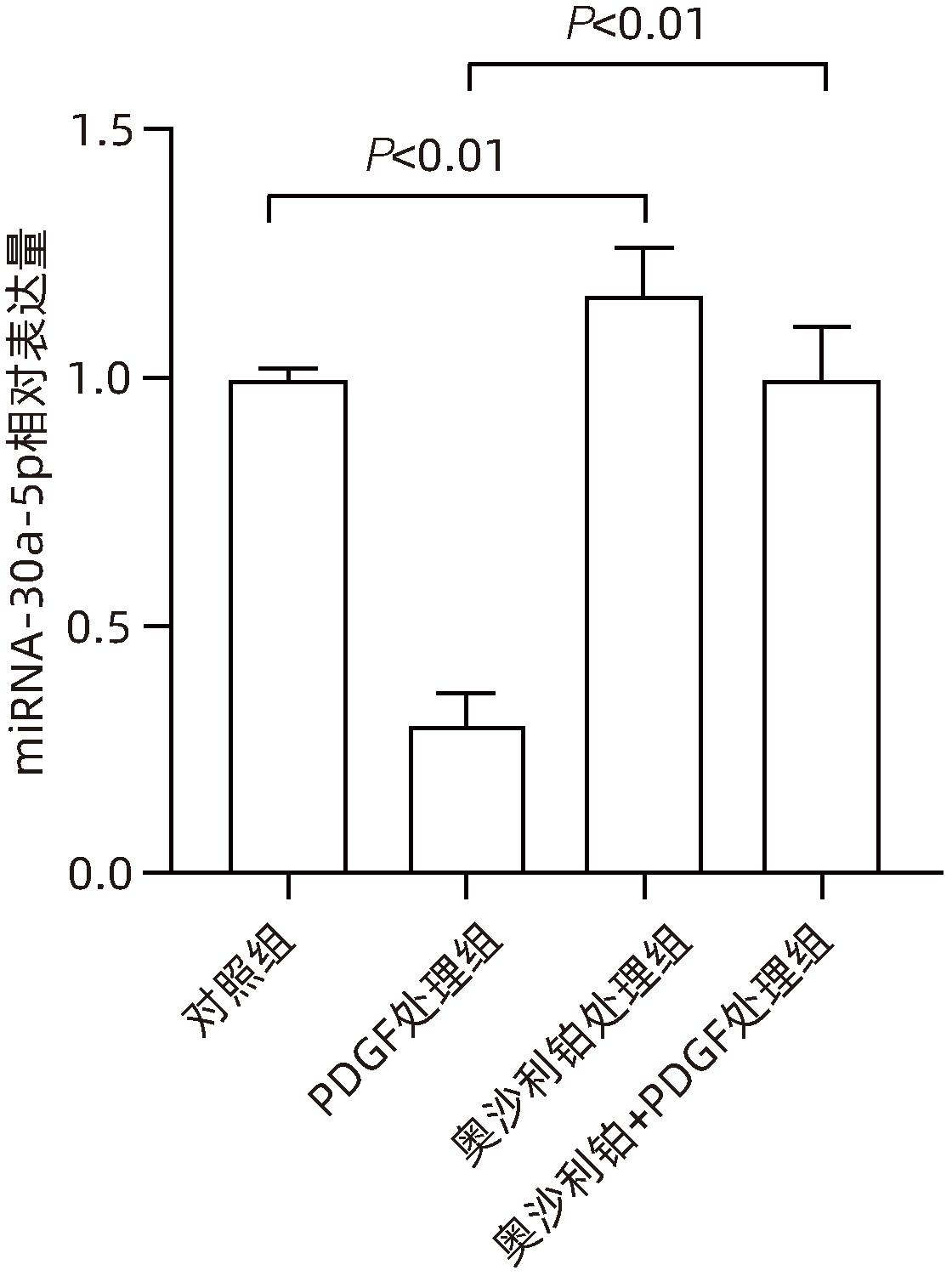
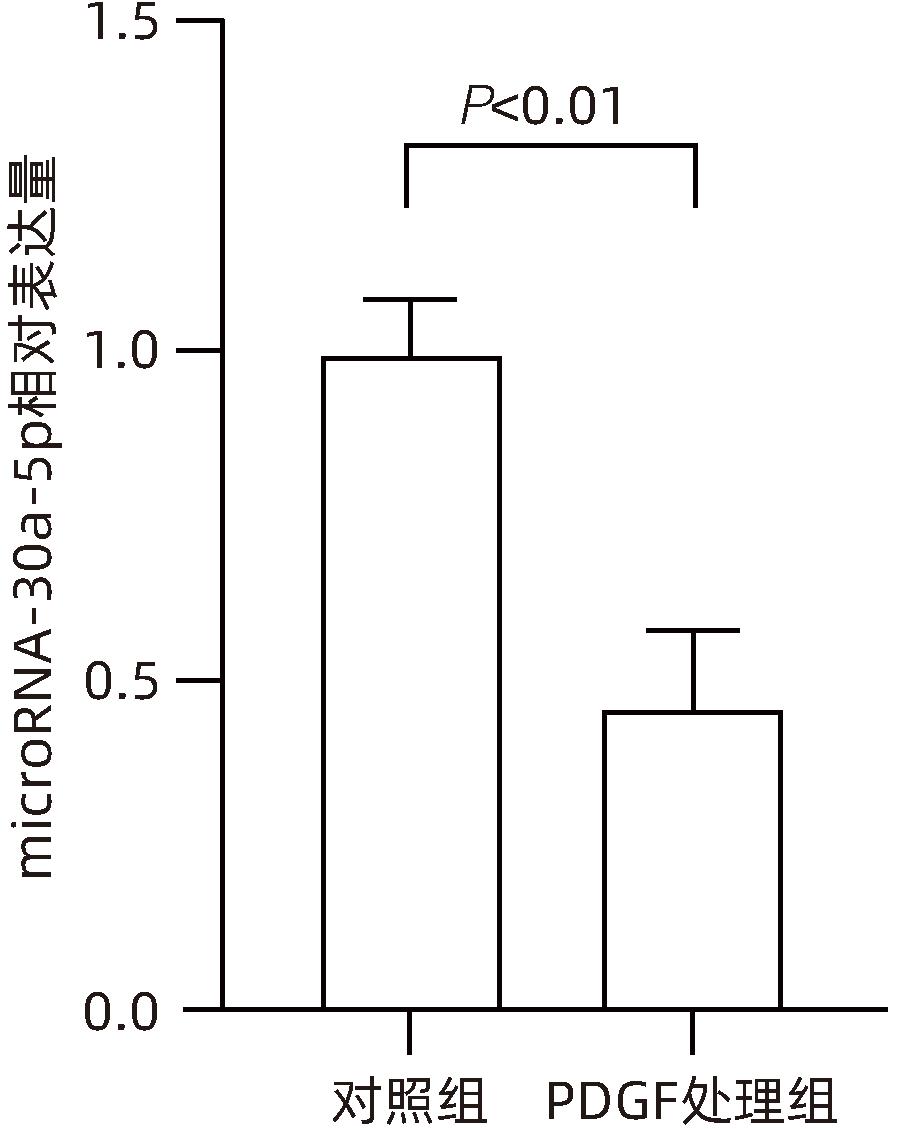
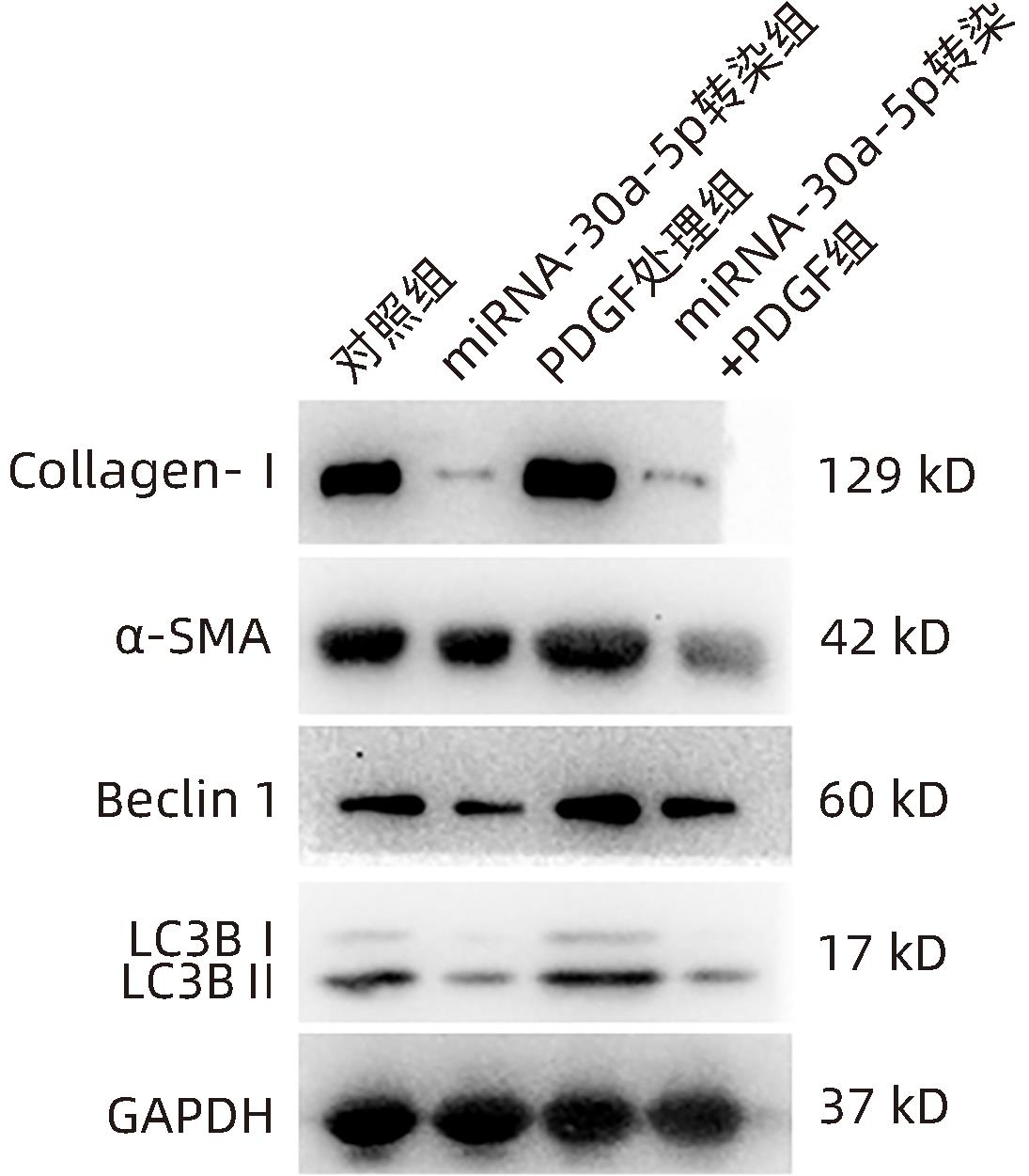
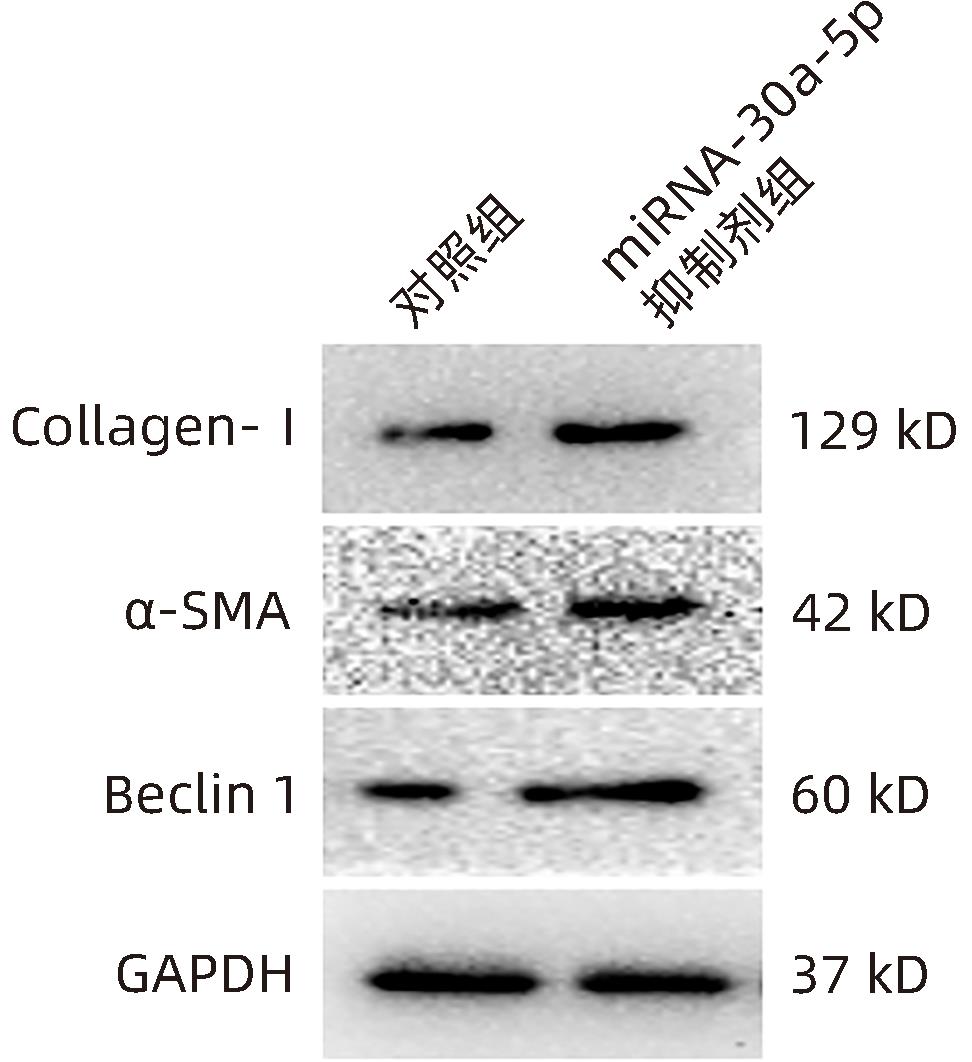
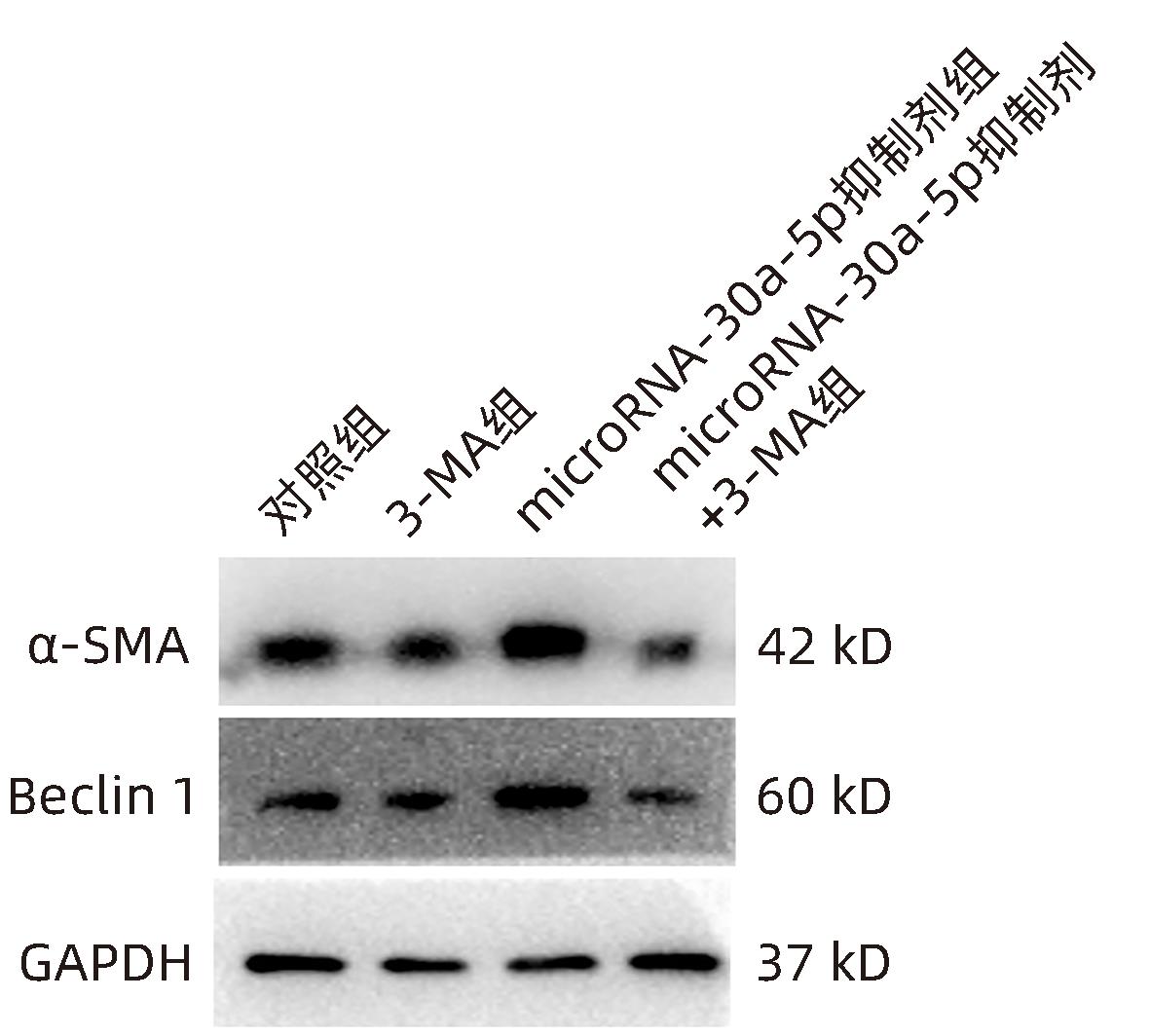
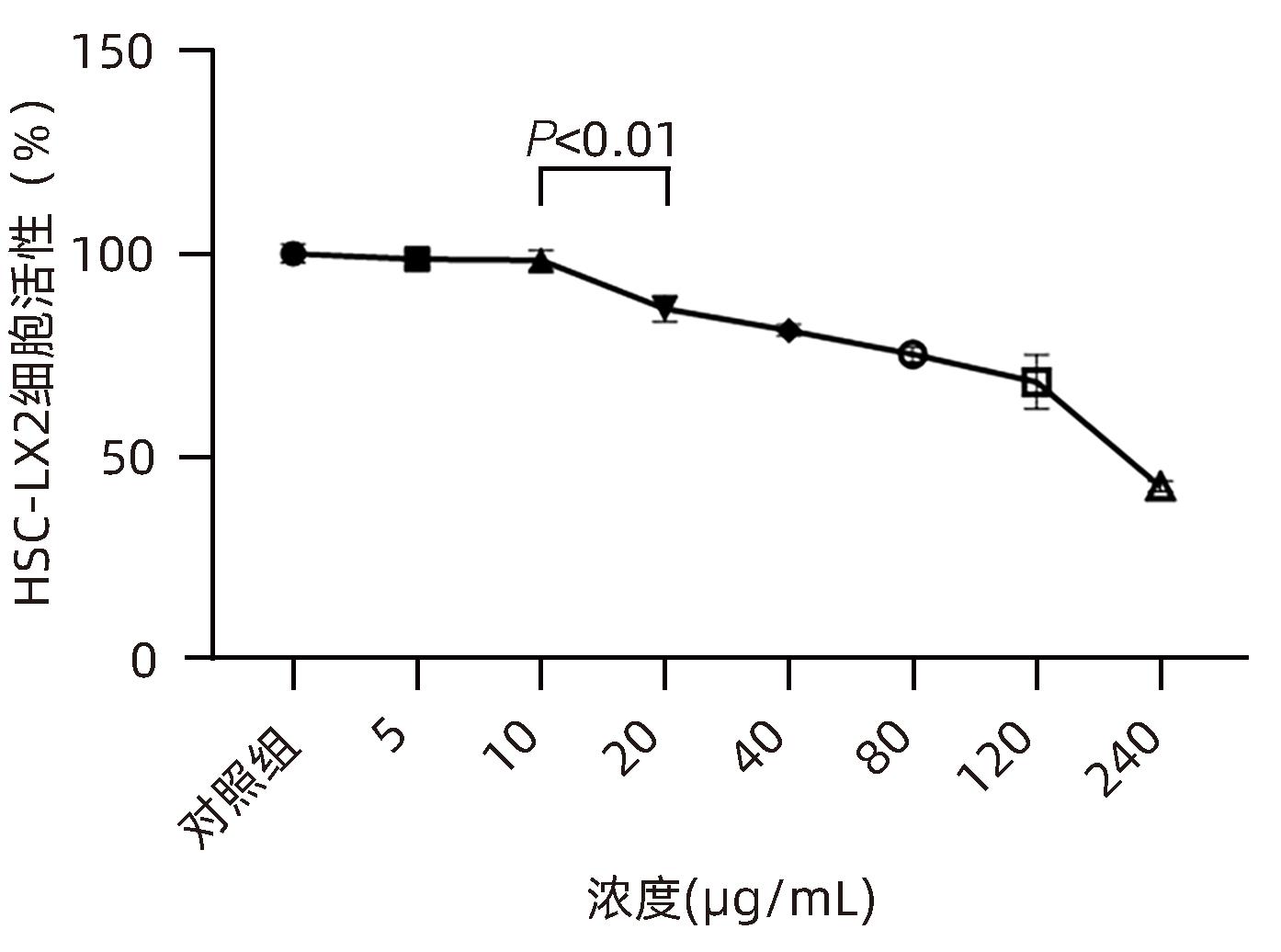
目的 观察奥沙利铂对肝星状细胞(HSC)活化的影响,进一步探讨奥沙利铂与microRNA-30a-5p及自噬的关系。 方法 培养HSC-LX2并做如下分组:(1)对照组、PDGF处理组、奥沙利铂处理组、奥沙利铂+PDGF处理组;(2)对照组、microRNA-30a-5p转染组,PDGF处理组、microRNA-30a-5p转染+PDGF处理组;(3)对照组、3-MA组、microRNA-30a-5p抑制剂组、microRNA-30a-5p抑制剂+3-MA组。应用免疫蛋白印迹技术(Western Blot)分析HSC活化相关蛋白Ⅰ型胶原(Collagen-Ⅰ)、抗α-平滑肌肌动蛋白(α-SMA)及HSC自噬相关蛋白Beclin 1、p62、LC3B的表达;溶酶体示踪剂和免疫荧光检测LC3B自噬小体的表达。实时荧光定量PCR(RT-PCR)检测microRNA-30a-5p表达水平。应用生物信息学技术预测microRNA-30a-5p在HSC中的潜在靶点。正态分布的计量资料两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 应用奥沙利铂处理细胞时,RT-PCR结果显示奥沙利铂处理组microRNA-30a-5p的表达水平高于对照组(P<0.01);Western Blot结果显示奥沙利铂处理组的HSC活化相关蛋白α-SMA、Collagen-Ⅰ及自噬相关蛋白Beclin 1、LC3BⅡ/Ⅰ水平均降低(P值均<0.001);免疫荧光结果显示奥沙利铂处理组自噬小体低于对照组(P<0.05)。进一步应用microRNA-30a-5p模拟类似物(mimic)转染HSC-LX2结果显示,与对照组相比,microRNA-30a-5p mimic组自噬相关蛋白Beclin 1和LC3BⅡ/Ⅰ的表达水平均降低(P值均<0.05);HSC活化相关蛋白Collagen-Ⅰ的表达水平亦降低(P<0.001);应用microRNA-30a-5p抑制剂转染HSC-LX2,Western Blot结果显示,与对照组比较,microRNA-30a-5p抑制剂组HSC活化相关蛋白Collagen-Ⅰ、α-SMA,自噬相关蛋白Beclin 1的表达水平均升高(t值分别为2.41、2.32、4.57,P值均<0.05)。Western Blot结果显示,与对照组相比,microRNA-30a-5p抑制剂组HSC自噬相关蛋白Beclin 1及活化相关蛋白α-SMA表达水平均升高(P值均<0.05),应用自噬抑制剂3-MA处理后,两组间上述蛋白表达无显著差异(P>0.05)。通过TargetScan、PicTar和miRanda生物信息软件分析表明,自噬相关蛋白Beclin 1是microRNA-30a-5p的潜在靶标。 结论 奥沙利铂可通过上调microRNA-30a-5p表达从而抑制HSC激活,为肝纤维化的治疗提供新的思路和作用靶点。 Abstract:Objective To investigate the effect of oxaliplatin on the activation of hepatic stellate cells (HSCs), as well as the association of oxaliplatin with microRNA-30a-5p and autophagy. Methods HSC-LX2 cells were cultured and divided into groups according to the following three protocols: control group, PDGF treatment group, oxaliplatin treatment group, oxaliplatin+PDGF treatment group; control group, microRNA-30a-5p transfection group, PDGF treatment group, microRNA-30a-5p transfection+PDGF treatment group; control group, 3-MA group, microRNA-30a-5p inhibitor group, microRNA-30a-5p inhibitor+3-MA group. Western Blot was used to measure the expression of HSC activation-related proteins (Collagen-I and alpha-smooth muscle actin [α- SMA]) and HSC autophagy-related proteins (Beclin-1, P62, and LC3B); LysoTracker staining and immunofluorescence assay were used to measure the expression of LC3B autophagosomes; RT-PCR was used to measure the expression level of microRNA-30a-5p; bioinformatics techniques were used to predict the potential targets of microRNA-30a-5p in HSCs. The independent-samples t test was used for comparison of normally distributed continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results After the cells were treated with oxaliplatin, RT-PCR results showed that the oxaliplatin treatment group had a significantly higher expression level of microRNA-30a-5p than the control group (P<0.01); Western Blot showed that the oxaliplatin treatment group had significant reductions in the expression levels of the HSC activation-related proteins α-SMA and Collagen-Ⅰ and the autophagy-related proteins Beclin 1 and LC3BⅡ/Ⅰ (all P<0.001); immunofluorescence assay showed that the oxaliplatin treatment group had a significantly lower number of autophagosomes than the control group (P<0.05). After HSC-LX2 cells were transfected with microRNA-30a-5p mimic, compared with the control group, the microRNA-30a-5p mimic group had significant reductions in the expression levels of the autophagy-related proteins Beclin 1 and LC3BⅡ/Ⅰ (P<0.05) and the HSC activation-related protein Collagen-Ⅰ (P<0.001); after HSC-LX2 cells were transfected with microRNA-30a-5p inhibitor, Western Blot showed that compared with the control group, the microRNA-30a-5p inhibitor group had significant increases in the expression levels of the HSC activation-related proteins Collagen-Ⅰ and α-SMA and the autophagy-related protein Beclin 1 (t=2.41, 2.32, and 4.57, all P<0.05). Western Blot showed that compared with the control group, the microRNA-30a-5p inhibitor group had significant increases in the expression levels of the HSC autophagy-related protein Beclin 1 and the HSC activation-related protein α-SMA (both P<0.05), and after the treatment with the autophagy inhibitor 3-MA, there were no significant differences in the expression of these proteins between the two groups (P>0.05). The bioinformatics analysis using TargetScan, PicTar, and miRanda databases showed that the autophagy-related protein Beclin-1 might be a potential target of miRNA-30a-5p. Conclusion Oxaliplatin can inhibit the activation of HSCs by upregulating the expression of microRNA-30a-5p, which provides new ideas and a new target for the treatment of liver fibrosis. -
Key words:
- Oxaliplatin /
- Hepatic Fibrosis /
- Hepatic Stellate Cells /
- MicroRNAs /
- Autophagy
-
-
[1] WEISKIRCHEN R, TACKE F. Liver fibrosis: From pathogenesis to novel therapies[J]. Dig Dis, 2016, 34( 4): 410- 422. DOI: 10.1159/000444556. [2] AYDIN MM, AKCALI KC. Liver fibrosis[J]. Turk J Gastroenterol, 2018, 29( 1): 14- 21. DOI: 10.5152/tjg.2018.17330. [3] BANSAL MB, CHAMROONKUL N. Antifibrotics in liver disease: Are we getting closer to clinical use?[J]. Hepatol Int, 2019, 13( 1): 25- 39. DOI: 10.1007/s12072-018-9897-3. [4] History GEERTS A., heterogeneity, biology developmental, and functions of quiescent hepatic stellate cells[J]. Semin Liver Dis, 2001, 21( 3): 311- 335. DOI: 10.1055/s-2001-17550. [5] ZHANG CY, YUAN WG, HE P, et al. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets[J]. World J Gastroenterol, 2016, 22( 48): 10512- 10522. DOI: 10.3748/wjg.v22.i48.10512. [6] YAMAGUCHI K, YANG L, MCCALL S, et al. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis[J]. Hepatology, 2008, 47( 2): 625- 635. DOI: 10.1002/hep.21988. [7] HERNÁNDEZ-GEA V, GHIASSI-NEJAD Z, ROZENFELD R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues[J]. Gastroenterology, 2012, 142( 4): 938- 946. DOI: 10.1053/j.gastro.2011.12.044. [8] SAITO T, KUMA A, SUGIURA Y, et al. Autophagy regulates lipid metabolism through selective turnover of NCoR1[J]. Nat Commun, 2019, 10( 1): 1567. DOI: 10.1038/s41467-019-08829-3. [9] TSUKAMOTO H. Cytokine regulation of hepatic stellate cells in liver fibrosis[J]. Alcohol Clin Exp Res, 1999, 23( 5): 911- 916. [10] LI JT, LIAO ZX, PING J, et al. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies[J]. J Gastroenterol, 2008, 43( 6): 419- 428. DOI: 10.1007/s00535-008-2180-y. [11] KITANO M, BLOOMSTON PM. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis[J]. J Clin Med, 2016, 5( 3): 38. DOI: 10.3390/jcm5030038. [12] GRIMSON A, FARH KKH, JOHNSTON WK, et al. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing[J]. Mol Cell, 2007, 27( 1): 91- 105. DOI: 10.1016/j.molcel.2007.06.017. [13] IORIO MV, CROCE CM. Causes and consequences of microRNA dysregulation[J]. Cancer J, 2012, 18( 3): 215- 222. DOI: 10.1097/PPO.0b013e318250c001. [14] LU RQ, ZHAO G, YANG YL, et al. Long noncoding RNA HOTAIRM1 inhibits cell progression by regulating miR-17-5p/PTEN axis in gastric cancer[J]. J Cell Biochem, 2019, 120( 4): 4952- 4965. DOI: 10.1002/jcb.27770. [15] FENG MH, LI JW, SUN HT, et al. Sulforaphane inhibits the activation of hepatic stellate cell by miRNA-423-5p targeting suppressor of fused[J]. Hum Cell, 2019, 32( 4): 403- 410. DOI: 10.1007/s13577-019-00264-2. [16] YU XX, RUAN Y, SHEN T, et al. Dexrazoxane protects cardiomyocyte from doxorubicin-induced apoptosis by modulating miR-17-5p[J]. Biomed Res Int, 2020, 2020: 5107193. DOI: 10.1155/2020/5107193. [17] HAZARI Y, BRAVO-SAN PEDRO JM, HETZ C, et al. Autophagy in hepatic adaptation to stress[J]. J Hepatol, 2020, 72( 1): 183- 196. DOI: 10.1016/j.jhep.2019.08.026. [18] APPS MG, CHOI EHY, WHEATE NJ. The state-of-play and future of platinum drugs[J]. Endocr Relat Cancer, 2015, 22( 4): R219- R233. DOI: 10.1530/ERC-15-0237. [19] TAN SF, SHI HJ, BA MC, et al. MiR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy[J]. Int J Mol Med, 2016, 37( 4): 1030- 1038. DOI: 10.3892/ijmm.2016.2492. [20] ZHU H, WU H, LIU XP, et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells[J]. Autophagy, 2009, 5( 6): 816- 823. DOI: 10.4161/auto.9064. [21] TSOCHATZIS EA, BOSCH J, BURROUGHS AK. Liver cirrhosis[J]. Lancet, 2014, 383( 9930): 1749- 1761. DOI: 10.1016/S0140-6736(14)60121-5. [22] POPOV Y, SCHUPPAN D. Targeting liver fibrosis: Strategies for development and validation of antifibrotic therapies[J]. Hepatology, 2009, 50( 4): 1294- 1306. DOI: 10.1002/hep.23123. [23] FRIEDMAN SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver[J]. Physiol Rev, 2008, 88( 1): 125- 172. DOI: 10.1152/physrev.00013.2007. [24] KISSELEVA T, BRENNER DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis[J]. J Gastroenterol Hepatol, 2007, 22( Suppl 1): S73- S78. DOI: 10.1111/j.1440-1746.2006.04658.x. [25] WANG J, CHU ESH, CHEN HY, et al. MicroRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway[J]. Oncotarget, 2015, 6( 9): 7325- 7338. DOI: 10.18632/oncotarget.2621. [26] OKADA H, HONDA M, CAMPBELL JS, et al. Inhibition of microRNA-214 ameliorates hepatic fibrosis and tumor incidence in platelet-derived growth factor C transgenic mice[J]. Cancer Sci, 2015, 106( 9): 1143- 1152. DOI: 10.1111/cas.12730. [27] KLINKHAMMER BM, FLOEGE J, BOOR P. PDGF in organ fibrosis[J]. Mol Aspects Med, 2018, 62: 44- 62. DOI: 10.1016/j.mam.2017.11.008. -



 PDF下载 ( 1223 KB)
PDF下载 ( 1223 KB)

 下载:
下载: