肝部分切除术后连续监测吲哚菁绿15分钟滞留率联合标准残肝体积对肝细胞癌患者肝功能不全的预测价值
DOI: 10.12449/JCH240615
Predictive value of continuous monitoring of indocyanine green retention rate at 15 minutes combined with standard residual liver volume for hepatic insufficiency in patients with hepatocellular carcinoma after partial hepatectomy
-
摘要:
目的 探讨肝部分切除术后连续性监测吲哚菁绿15分钟滞留率(ICG-R15)联合标准残肝体积(SRLV)对术后肝功能不全发生率的预测价值。 方法 收集天津市第一中心医院肝胆外科2016年11月—2017年5月收治的70例肝细胞癌患者的临床资料。根据患者术后是否发生肝功能不全的情况,分为肝功能良好组(n=56)与肝功能不全组(n=14)。根据术前肝功能评估及强化CT计算切除肝体积和剩余肝体积以及肝脏三维重建,术中B超定位决定手术方案,根据术中情况决定采用选择性肝门入肝血流阻断或间断全肝门阻断,CUSA联合双极滴水电凝镊离断肝实质。计算SRLV并连续监测患者ICG-R15。计量资料两组间比较采用成组t检验;计数资料组间比较采用χ2检验;采用受试者工作特征曲线(ROC曲线)下面积(AUC)评估预测术后肝功能不全的准确性。多因素Logistic回归分析建立术后肝功能不全的预测模型,制订SLRV联合术后ICG-R15动态监测对术后肝功能不全的诊断标准。 结果 两组术前以及术后即时、3 d、5 d的ICG-R15,以及SRLV、Child分级比较,差异均有统计学意义(P值均<0.05)。术前、术后即时、术后3 d、术后5 d 患者术后肝功能不全发生率均随ICG-R15升高而升高(P值均<0.001)。进一步两两比较,术前、术后即时、术后3 d、术后5 d ICG-R15>20%组与其他两组患者肝功能不全发生率比较,差异均有统计学意义(P值均<0.001);术后即时,ICG-R15<10%组与10%≤ICG-R15≤20%组肝功能不全发生率比较,差异有统计学意义(P<0.001)。术前ICG-R15、术后即时ICG-R15、术后3 d ICG-R15、术后5 d ICG-R15预测术后肝功能不全的AUC分别为0.790、0.857、0.855、0.870,术后即时、3 d、5 d ICG-R15的AUC均大于术前,差异均有统计学意义(P值均<0.05)。多因素分析结果显示,SRLV以及ICG-R15术后动态监测(术后即时、术后3 d、术后5 d)水平升高均为术后肝功能不全的独立危险因素,术前BMI升高则为独立保护因素(P值均<0.05)。建立多因素Logistic回归预测模型,依据预测模型分别对术后(术后即时、术后3 d、术后5 d)肝功能不全进行预测,ROC曲线显示术后即时、术后3 d、术后5 d预测模型的AUC分别为0.963、0.967、0.967(P值均<0.01)。制订SLRV联合术后ICG-R15动态监测对于术后肝功能不全的诊断标准:SLRV>1 240 mL/m2、术后即时ICG-R15>20%、术后3 d或5 d ICG-R15>25%,符合其中任意一项即可诊断术后肝功能不全,灵敏度100%,特异度60.71%,符合度68.57%。 结论 连续性监测术前及术后ICG-R15水平对于术后肝功能不全的预估均具有指导意义,术后5 d ICG-R15的准确性相对最高;SRLV联合术后ICG-R15动态检测能有效预测肝切除术后肝功能不全的发生,可指导临床医生预估肝癌患者术后肝功能不全的发生,并尽早进行临床干预。 Abstract:Objective To investigate the value of continuous monitoring of indocyanine green retention rate at 15 minutes (ICG-R15) combined with standard residual liver volume (SRLV) in predicting hepatic insufficiency after partial hepatectomy. Methods Clinical data and SRLV data were collected from 70 patients with hepatocellular carcinoma who were admitted to Department of Hepatobiliary Surgery, Tianjin First Central Hospital, from November 2016 to May 2017. According to the presence or absence of hepatic insufficiency after surgery, the patients were divided into good liver function group with 56 patients and hepatic insufficiency group with 14 patients. Based on preoperative liver function evaluation and contrast-enhanced CT scans, resected liver volume and residual liver volume were calculated, and three-dimensional reconstruction of the liver was performed. Intraoperative ultrasound localization was performed to determine the surgical regimen, and selective hepatic inflow occlusion or intermittent hepatic portal occlusion was selected based on intraoperative conditions. CUSA combined with BIPOLAR drip electric coagulation forceps were used for the partition of liver parenchyma. SRLV was calculated, and ICG-R15 was monitored continuously. The independent-samples t test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups; the area under the ROC curve (AUC) was used to investigate the accuracy in predicting hepatic insufficiency after surgery. A multivariate Logistic regression analysis was used to establish a predictive model for postoperative hepatic insufficiency, and diagnostic criteria were developed for SLRV combined with postoperative ICG-R15 dynamic monitoring in the diagnosis of postoperative hepatic insufficiency. Results There were significant differences between the two groups in ICG-R15 before surgery, immediately after surgery, and on days 3 and 5 after surgery, as well as significant differences in SRLV and Child class (all P<0.05). The incidence rate of postoperative hepatic insufficiency increased with the increase in ICG-R15 before surgery, immediately after surgery, and on days 3 and 5 after surgery (all P<0.001). Further comparison between two groups showed that there was a significant difference in the incidence rate of hepatic insufficiency between the ICG-R15>20% group and the other two groups before surgery, immediately after surgery, and on days 3 and 5 after surgery (all P<0.001), and there was a significant difference in the incidence rate of hepatic insufficiency between the ICG-R15<10% group and the 10%≤ICG-R15≤20% group immediately after surgery (P<0.001). ICG-R15 before surgery, ICG-R15 immediately after surgery, ICG-R15 on day 3 after surgery, and ICG-R15 on day 5 after surgery had an AUC of 0.790, 0.857, 0.855, and 0.870, respectively, in predicting postoperative hepatic insufficiency, and ICG-R15 immediately after surgery and on days 3 and 5 after surgery had a significantly larger AUC than ICG-R15 before surgery (all P<0.05). The multivariate analysis showed that increases in SRLV and postoperative ICG-R15 dynamic monitoring (immediately after surgery and on days 3 and 5 after surgery) were independent risk factors for postoperative hepatic insufficiency, while increased body mass index before surgery was an independent protective factor (all P<0.05). A multivariate Logistic regression predictive model was established and was used to predict hepatic insufficiency after surgery (immediately after surgery and on days 3 and 5 after surgery), and the ROC curve analysis showed that the model had an AUC of 0.963, 0.967, and 0.967, respectively, in predicting hepatic insufficiency immediately after surgery and on days 3 and 5 after surgery (all P<0.01). Diagnostic criteria were developed for SLRV combined with postoperative ICG-R15 dynamic monitoring in the diagnosis of postoperative hepatic insufficiency, i.e., SLRV>1 240 mL/m2, ICG-R15>20% immediately after surgery, or ICG-R15>25% on day 3 or 5 after surgery, and postoperative hepatic insufficiency could be diagnosed if a patient met any one criterion. These diagnostic criteria had a sensitivity of 100%, a specificity of 60.71%, and a conformity degree of 68.57%. Conclusion Continuous monitoring of ICG-R15 before and after surgery is of guiding significance for predicting postoperative hepatic insufficiency, and ICG-R15 on day 5 after surgery has the highest accuracy. SRLV combined with postoperative ICG-R15 dynamic monitoring can effectively predict the onset of hepatic insufficiency after hepatectomy and can guide clinicians to predict the onset of postoperative hepatic insufficiency in patients with liver cancer and perform clinical intervention as soon as possible. -
Key words:
- Liver Neoplasms /
- Hepatectomy /
- Indocyanine Green /
- Hepatic Insufficiency
-
表 1 术后肝功能良好组与肝功能不全组的临床指标比较
Table 1. Comparison of clinical indexes between normal liver function group and liver insufficiency group after operation
项目 肝功能良好组(n=56) 肝功能不全组(n=14) 统计值 P值 年龄(岁) 54.82±10.87 56.57±5.08 t=0.880 0.383 性别(例) χ2=0.036 0.850 男 36 10 女 20 4 Child分级(例) χ2=23.414 <0.001 A级 50 4 B级 6 10 肝脏体积(cm³) 1 667.83±537.92 1 978.70±538.96 t=1.933 0.057 SRLV(mL/m2) 1 132.38±199.66 1 374.62±173.28 t=4.160 <0.001 切除肝体积(cm3) 514.03±460.72 696.89±560.07 t=1.271 0.208 手术时间(min) 223.30±44.13 249.71±50.03 χ2=1.951 0.055 出血量(mL) 419.64±176.24 500.00±203.81 χ2=1.479 0.144 输血量(mL) 321.43±173.43 428.57±209.13 χ2=1.983 0.051 ICG-R15 术前 5.73%±4.31% 18.02%±13.48% t=3.368 0.005 术后即时 12.36%±7.41% 32.54%±18.67% t=3.966 0.001 术后3 d 11.51%±8.76% 34.73%±18.49% t=4.571 <0.001 术后5 d 9.60%±7.00% 25.34%±11.49% t=4.901 <0.001 表 2 术后连续性监测ICG-R15水平与肝功能不全发生率的关系
Table 2. Relationship between continuous monitoring of ICG-R15 level and incidence of liver insufficiency after surgery
组别 例数 肝功能良好[例(%)] 肝功能不全[例(%)] χ2值 P值 术前 22.374 <0.001 ICG-R15<10%组 54 48(88.9) 6(11.1)1) 10%≤ICG-R15≤20%组 10 8(80.0) 2(20.0)1) ICG-R15>20%组 6 0(0.0) 6(100.0) 术后即时 28.024 <0.001 ICG-R15<10%组 32 32(100.0) 0(0.0)1)2) 10%≤ICG-R15≤20%组 22 18(81.8) 4(18.2)1) ICG-R15>20%组 16 6(37.5) 10(62.5) 术后3 d 14.810 <0.001 ICG-R15<10%组 32 30(93.8) 2(6.3)1) 10%≤ICG-R15≤20%组 18 16(88.9) 2(11.1)1) ICG-R15>20%组 20 10(50.0) 10(50.0) 术后5 d 21.159 <0.001 ICG-R15<10%组 38 36(94.7) 2(5.3)1) 10%≤ICG-R15≤20%组 16 14(87.5) 2(12.5)1) ICG-R15>20%组 16 6(37.5) 10(62.5) 注:与ICG-R15>20%组比较,1)P<0.001;与10%≤ICG-R15≤20%组比较,2)P<0.001。 表 3 术前、术后连续性监测ICG-R15的AUC及95%CI
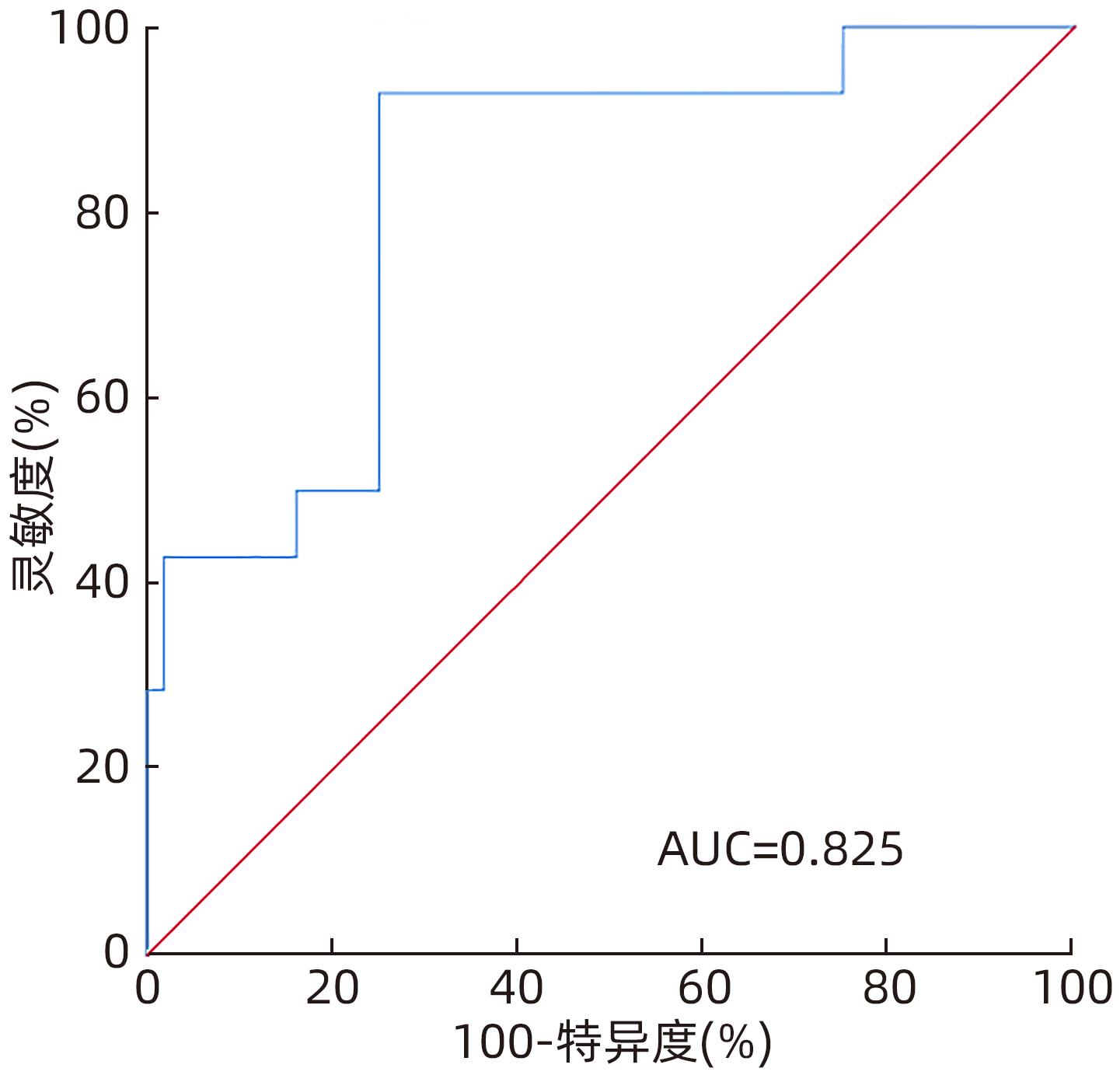
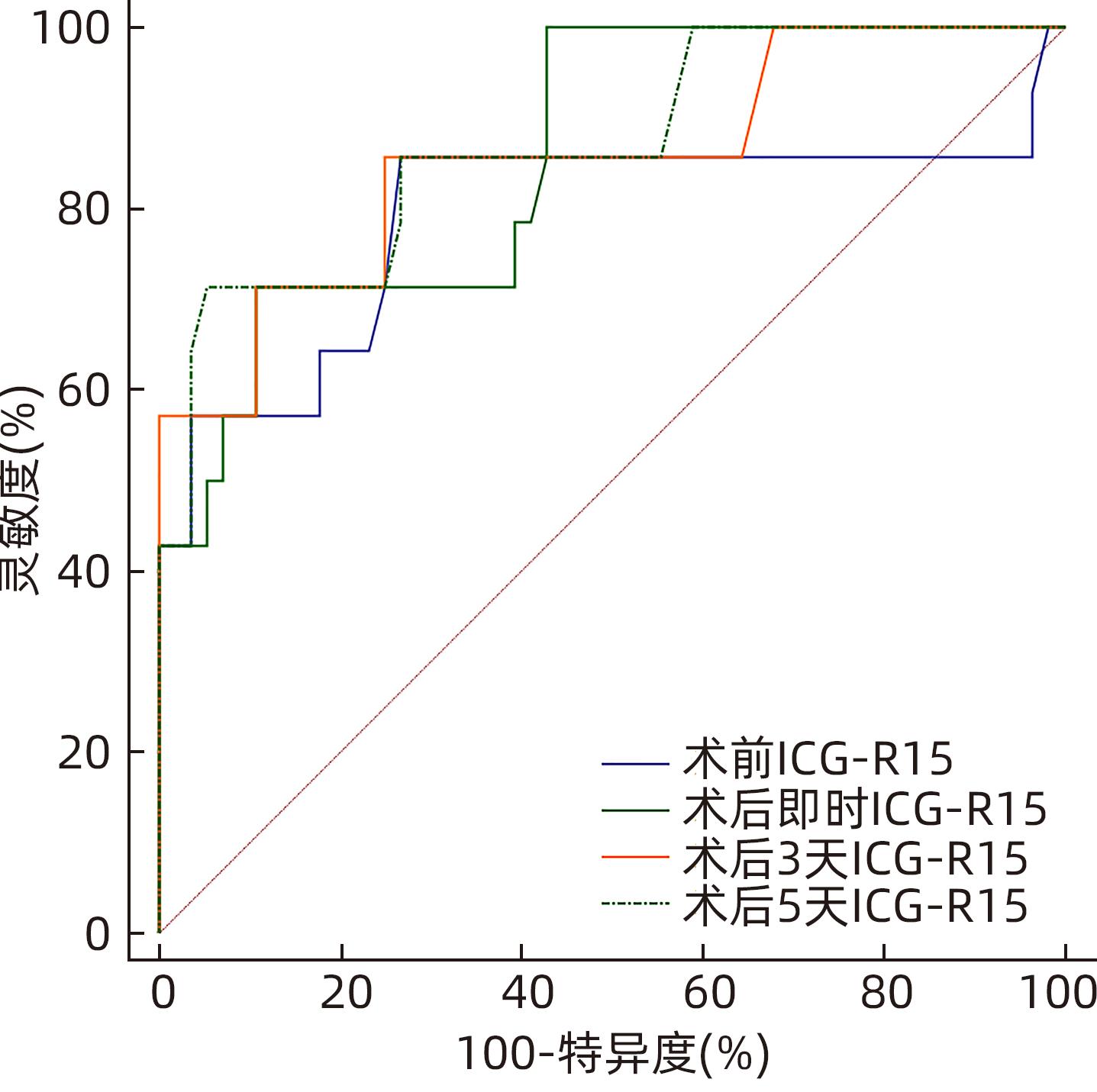
Table 3. The level of ICG-R15 and AUC and 95%CI were continuously monitored before and after operation
ICG-R15 AUC(95%CI) 截断值(%) 灵敏度(%) 特异度(%) 术前 0.790(0.676~0.878) 6.10 85.71 73.21 术后即时 0.857(0.752~0.929) 17.50 71.43 89.29 术后3 d 0.855(0.750~0.927) 14.30 85.71 75.00 术后5 d 0.870(0.768~0.938) 25.20 71.43 94.64 表 4 肝癌患者肝部分切除术后肝功能不全的多因素Logistic 回归分析
Table 4. Multivariate Logistic regression analysis of postoperative hepatic insufficiency
项目 OR(95%CI) P值 术后即时预测模型 术前BMI 0.448(0.246~0.816) <0.01 术后即时ICG-R15 1.118(1.007~1.241) <0.01 SRLV 1.020(1.004~1.036) <0.01 术后3 d预测模型 术前BMI 0.451(0.241~0.843) <0.01 术后3天ICG-R15 1.112(1.018~1.213) <0.01 SRLV 1.021(1.003~1.038) <0.01 术后5 d预测模型 术前BMI 0.464(0.253~0.849) <0.01 术后5天ICG-R15 1.150(1.026~1.288) <0.01 SRLV 1.017(1.002~1.033) <0.01 表 5 术后动态监测ICG-R15对术后肝功能不全的诊断折点
Table 5. Postoperative dynamic monitoring of ICG-R15 in the diagnosis of hepatic insufficiency
ICG-R15 诊断折点 灵敏度(%) 特异度(%) 约登指数1) 术后即时 >20.80% 71.4 89.3 0.607 术后3 d >14.35% 85.7 75.0 0.607 >25.05% 71.4 89.3 0.607 术后5 d >25.35% 71.4 94.6 0.660 >24.35% 71.4 92.9 0.643 >23.35% 71.4 91.1 0.625 >19.35% 71.4 89.3 0.607 >25.55% 64.3 96.4 0.607 注:1)列举约登指数较大的诊断折点。 表 6 SLRV联合术后ICG-R15动态监测对术后肝功能不全的诊断标准
Table 6. SLRV combined with ICG-R15 dynamic monitoring for the diagnosis of postoperative hepatic insufficiency
诊断标准 诊断条件 标准一 符合以下任意一项或多项: (1)SLRV>1 240 mL/m2; (2)术后即时ICG-R15>20%; (3)术后3 d或5 d ICG-R15>25% 标准二 符合以下所有项: (1)SLRV>1 240 mL/m2; (2)术后即时ICG-R15>20%; (3)术后3 d或5 d ICG-R15>25% 表 7 SLRV联合术后ICG-R15动态监测对术后肝功能不全的诊断性能评价
Table 7. Evaluation of SLRV combined with ICG-R15 dynamic monitoring in the diagnosis of postoperative hepatic insufficiency
诊断标准 肝功能不全(例) 肝功能良好(例) 灵敏度(%) 漏诊率(%) 特异度(%) 误诊率(%) 符合度(%) 标准一1) 14 34 100.00 0.00 60.71 39.29 68.57 标准二2) 9 56 64.29 35.71 100.00 0.00 92.85 注:1)另有22例符合标准一,但不符合金标准;2)另有5例符合金标准,但不符合标准2。 -
[1] RAHBARI NN, GARDEN OJ, PADBURY R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery(ISGLS)[J]. Surgery, 2011, 149( 5): 713- 724. DOI: 10.1016/j.surg.2010.10.001. [2] HAEGELE S, REITER S, WANEK D, et al. Perioperative non-invasive indocyanine green-clearance testing to predict postoperative outcome after liver resection[J]. PLoS One, 2016, 11( 11): e0165481. DOI: 10.1371/journal.pone.0165481. [3] DU ZG, LI B, FENG X, et al. Combined indocyanine green test and standard remnant liver volume to predict post-hepatectomy hepatic insufficiency for the patients with hepatocellular carcinoma[J]. Chin J Surg, 2010, 48( 3): 189- 192. DOI: 10.3760/cma.j.issn.0529-5815.2010.03.010.杜正贵, 李波, 冯曦, 等. 吲哚氰绿排泄试验及标准余肝体积与肝癌切除术后肝功能不全的相关性研究[J]. 中华外科杂志, 2010, 48( 3): 189- 192. DOI: 10.3760/cma.j.issn.0529-5815.2010.03.010. [4] DONG JH, ZHENG SS, CHEN XP, et al. Consensus on evaluation of hepatic functional reserve before hepatectomy(2011 edition)[J]. Chin J Dig Surg, 2011, 10( 1): 20- 25. DOI: 10.3760/cma.j.issn.1673-9752.2011.01.006.董家鸿, 郑树森, 陈孝平, 等. 肝切除术前肝脏储备功能评估的专家共识(2011版)[J]. 中华消化外科杂志, 2011, 10( 1): 20- 25. DOI: 10.3760/cma.j.issn.1673-9752.2011.01.006. [5] XU LN, XU YY, GAO DW. Impact of operative and peri-operative factors on the long-term prognosis of primary liver cancer patients undergoing hepatectomy[J]. J Huazhong Univ Sci Technolog Med Sci, 2016, 36( 4): 523- 528. DOI: 10.1007/s11596-016-1619-2. [6] TAKAHASHI K, KUROKAWA T, OSHIRO Y, et al. Postoperative decrease in platelet counts is associated with delayed liver function recovery and complications after partial hepatectomy[J]. Tohoku J Exp Med, 2016, 239( 1): 47- 55. DOI: 10.1620/tjem.239.47. [7] LIU Y, CHEN ZL, YU XX, et al. Risk factors for hepatic insufficiency after major hepatectomy in non-cirrhotic patients[J]. Asian J Surg, 2021, 44( 10): 1324- 1325. DOI: 10.1016/j.asjsur.2021.06.046. [8] APERS T, HENDRIKX B, BRACKE B, et al. Parenchymal-sparing hepatectomy with hepatic vein resection and reconstruction[J]. Acta Chir Belg, 2022, 122( 5): 334- 340. DOI: 10.1080/00015458.2021.1915021. [9] SØREIDE JA, DESHPANDE R. Post hepatectomy liver failure(PHLF)-Recent advances in prevention and clinical management[J]. Eur J Surg Oncol, 2021, 47( 2): 216- 224. DOI: 10.1016/j.ejso.2020.09.001. [10] HAYASHI H, BEPPU T, OKABE H, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy[J]. Surgery, 2015, 157( 1): 20- 26. DOI: 10.1016/j.surg.2014.06.013. [11] MOON YJ, KIM SH, KIM JW, et al. Comparison of postoperative coagulation profiles and outcome for sugammadex versus pyridostigmine in 992 living donors after living-donor hepatectomy[J]. Medicine, 2018, 97( 11): e0129. DOI: 10.1097/MD.0000000000010129. [12] DE RUDDER M, DILI A, STÄRKEL P, et al. Critical role of LSEC in post-hepatectomy liver regeneration and failure[J]. Int J Mol Sci, 2021, 22( 15): 8053. DOI: 10.3390/ijms22158053. [13] ZHANG ZM, OUYANG GX, WANG P, et al. Safe standard remnant liver volume after hepatectomy in HCC patients in different stages of hepatic fibrosis[J]. BMC Surg, 2021, 21( 1): 57. DOI: 10.1186/s12893-021-01065-x. [14] YOSHIDA M, SHIRAISHI S, SAKAMOTO F, et al. Assessment of hepatic functional regeneration after hepatectomy using(99m)Tc-GSA SPECT/CT fused imaging[J]. Ann Nucl Med, 2014, 28( 8): 780- 788. DOI: 10.1007/s12149-014-0872-3. [15] FUNG J, POON RTP, YU WC, et al. Use of liver stiffness measurement for liver resection surgery: Correlation with indocyanine green clearance testing and post-operative outcome[J]. PLoS One, 2013, 8( 8): e72306. DOI: 10.1371/journal.pone.0072306. [16] CHEN ZS, LIN KC, LIU JF. Application of three-dimensional visualization in surgical operation for primary liver cancer[J]. J Clin Hepatol, 2022, 38( 3): 505- 509. DOI: 10.3969/j.issn.1001-5256.2022.03.003.陈昭硕, 林科灿, 刘景丰. 三维可视化技术在原发性肝癌外科手术中的应用[J]. 临床肝胆病杂志, 2022, 38( 3): 505- 509. DOI: 10.3969/j.issn.1001-5256.2022.03.003. [17] BOWEN SR, CHAPMAN TR, BORGMAN J, et al. Measuring total liver function on sulfur colloid SPECT/CT for improved risk stratification and outcome prediction of hepatocellular carcinoma patients[J]. EJNMMI Res, 2016, 6( 1): 57. DOI: 10.1186/s13550-016-0212-9. [18] ETRA JW, SQUIRES MH 3rd, FISHER SB, et al. Early identification of patients at increased risk for hepatic insufficiency, complications and mortality after major hepatectomy[J]. HPB, 2014, 16( 10): 875- 883. DOI: 10.1111/hpb.12270. [19] GAO YY, ZHANG X, LI FH, et al. Measurement of glycosylated albumin and its application value in liver cirrhosis patients with different Child-Pugh classes[J]. J Clin Hepatol, 2022, 38( 2): 347- 351. DOI: 10.3969/j.issn.1001-5256.2022.02.018.高艳颖, 张旭, 李凤慧, 等. 不同Child-Pugh分级肝硬化患者糖化白蛋白的测定及其应用价值[J]. 临床肝胆病杂志, 2022, 38( 2): 347- 351. DOI: 10.3969/j.issn.1001-5256.2022.02.018. [20] ZHAO D, YE JD, LI HL, et al. Application of liver three-dimensional visualized reconstruction technique in hepatectomy for children with compli-cated hepatoblastoma[J]. J Clin Hepatol, 2021, 37( 9): 2130- 2135. DOI: 10.3969/j.issn.1001-5256.2021.09.025.赵頔, 叶进冬, 李华丽, 等. 肝脏三维可视化重建技术在儿童复杂肝母细胞瘤肝切除术中的应用价值[J]. 临床肝胆病杂志, 2021, 37( 9): 2130- 2135. DOI: 10.3969/j.issn.1001-5256.2021.09.025. -



 PDF下载 ( 797 KB)
PDF下载 ( 797 KB)

 下载:
下载: