冷冻消融联合卡瑞利珠单抗治疗肝细胞癌的效果及安全性分析
DOI: 10.12449/JCH240616
Efficacy and safety of cryoablation combined with Camrelizumab monoclonal antibody in treatment of hepatocellular carcinoma
-
摘要:
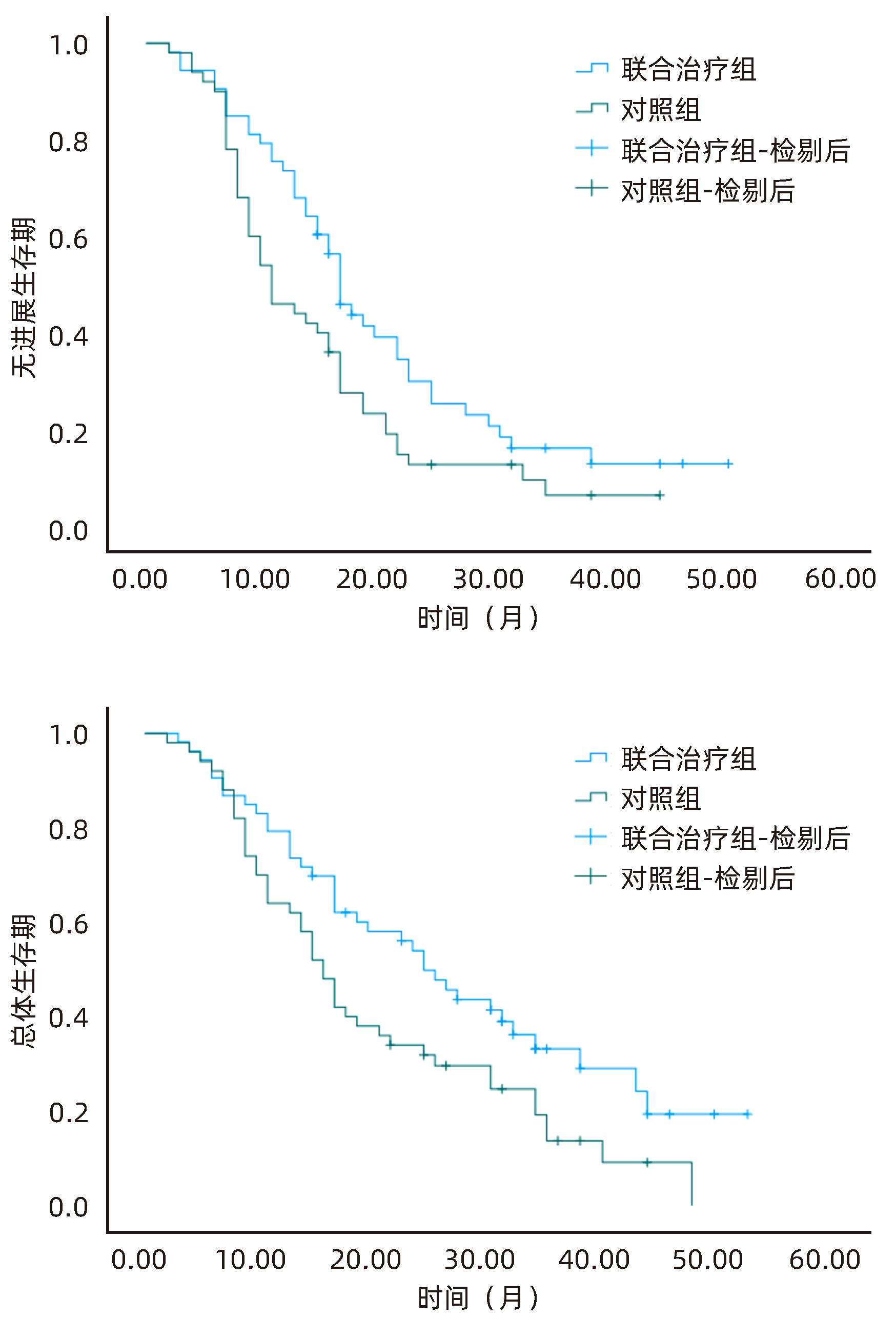
目的 探讨分析冷冻消融联合抑制剂卡瑞利珠单抗治疗肝细胞癌(HCC)的有效性和安全性。 方法 选取2020年6月—2023年6月河北医科大学第一医院收治的HCC患者103例为研究对象,将患者随机分为联合治疗组(53例)和对照组(50例)。对照组患者接受经皮氩氦刀冷冻消融术治疗,联合治疗组患者接受经皮氩氦刀冷冻消融术联合卡瑞利珠单抗治疗。比较两组患者近期疗效、治疗前后T淋巴细胞亚群变化、肝功能及AFP变化、随访无进展生存期及总体生存期。符合正态分布的计量资料两组间比较采用成组t检验;计数资料两组间比较采用χ2检验。Kaplan-Meier法绘制生存曲线,Log-rank检验比较两组生存时间差异。 结果 联合治疗组患者总缓解率、疾病控制率均明显高于对照组(χ2值分别为4.156、4.348,P值分别为0.042、0.037)。联合治疗组患者治疗后CD3+、CD4+T淋巴细胞百分比及CD4+/CD8+值较治疗前均明显升高(P<0.05),CD8+T淋巴细胞百分比较治疗前明显降低(P<0.05),而对照组患者治疗前、后T淋巴细胞亚群均无明显变化(P值均>0.05),且治疗后联合治疗组CD3+、CD4+T淋巴细胞百分比及CD4+/CD8+值均明显高于对照组(P值均<0.05),CD8+细胞百分比明显低于对照组(P<0.05)。两组患者治疗后ALT、AST、AFP水平较治疗前均明显降低(P值均<0.05),Alb水平较治疗前明显升高(P<0.05),且联合治疗组患者治疗后ALT、AST、AFP水平明显低于对照组(P值均<0.05),Alb水平明显高于对照组(P<0.05)。两组患者Ⅲ~Ⅳ级(中重度)不良反应发生率比较差异无统计学意义(P>0.05)。联合治疗组患者无进展生存中位时间(21.32个月 vs 15.31个月)、总体生存中位时间(28.36个月 vs 20.75个月)均明显优于对照组(χ2值分别为4.689、5.030,P值分别为0.030、0.025)。 结论 氩氦刀冷冻消融联合卡瑞利珠单抗可有效提升近期疗效,改善机体免疫功能,延长生存时间,且治疗安全性良好。 Abstract:Objective To investigate the efficacy and safety of cryoablation combined with Camrelizumab monoclonal antibody in the treatment of hepatocellular carcinoma (HCC). Methods A total of 103 HCC patients who were admitted to our hospital from June 2020 to June 2023 were enrolled and randomly divided into combined treatment group with 53 patients and control group with 50 patients. The patients in the control group received percutaneous argon-helium cryoablation, and those in the combined treatment group received percutaneous argon-helium cryoablation combined with Camrelizumab monoclonal antibody. The two groups were compared in terms of short-term response, changes in T lymphocyte subsets after treatment, changes in liver function and alpha-fetoprotein (AFP) after treatment, and progression-free survival and overall survival during follow-up. The t-test was used for comparison of normally distributed continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison of survival time between the two groups. Results The combined treatment group had significantly higher overall response rate and disease control rate than the control group (χ2=4.156 and 4.348, P=0.042 and 0.037). After treatment, the combined treatment group had significant increases in the percentages of CD3+ and CD4+ T lymphocytes and CD4+/CD8+ ratio (P<0.05) and a significant reduction in the percentage of CD8+ T lymphocytes (P<0.05), while the control group had no significant changes in T lymphocyte subsets after treatment (P>0.05), and compared with the control group after treatment, the combined treatment group had significantly higher percentages of CD3+ and CD4+ T lymphocytes and CD4+/CD8+ ratio (all P<0.05) and a significantly lower percentage of CD8+ T lymphocytes (P<0.05). After treatment, both groups had significant reductions in the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and AFP (all P<0.05) and a significant increase in the level of albumin (Alb) (P>0.05), and compared with the control group after treatment, the combined treatment group had significantly lower levels of ALT, AST, and AFP (all P<0.05) and a significantly higher level of Alb (P<0.05). There were no significant differences in the incidence rates of grade Ⅲ—Ⅳ (moderate to severe) adverse reactions between the two groups (P>0.05). Compared with the control group, the combined treatment group had significantly better median progression-free survival (21.32 months vs 15.31 months, χ2=4.689, P=0.030) and median overall survival (28.36 months vs 20.75 months, χ2=5.030, P=0.025). Conclusion Argon-helium cryoablation combined with Camrelizumab monoclonal antibody can effectively improve short-term response, enhance immune function, and prolong survival time, with a favorable safety profile. -
Key words:
- Carcinoma, Hepatocellular /
- Cryosurgery /
- Immune Checkpoint Inhibitors
-
表 1 两组患者一般资料比较
Table 1. Comparison of general data between two groups of patients
项目 联合治疗组(n=53) 对照组(n=50) 统计值 P值 性别(例) χ2=0.172 0.679 男 37 33 女 16 17 年龄(岁) 64.59±7.21 65.26±8.35 t=0.437 0.663 肿瘤最大径(cm) 8.39±2.37 8.48±2.10 t=0.204 0.839 Child-Pugh分级(例) χ2=0.006 0.938 A级 41 39 B级 12 11 CNLC分期(例) χ2=0.329 0.566 Ⅱa~Ⅱb期 31 32 Ⅲa~Ⅲb期 22 18 肿瘤个数(例) χ2=0.160 0.689 单发 40 36 多发 13 14 其他综合性抗癌方案(例) 放疗 7 9 χ2=0.397 0.529 化疗 14 15 χ2=0.119 0.731 TACE 7 10 χ2=0.785 0.376 无 29 24 χ2=0.465 0.495 注:TACE,经肝动脉化疗栓塞。 表 2 两组患者T淋巴细胞亚群变化
Table 2. Changes of T lymphocyte subsets in two groups of patients
组别 例数 CD3+ T淋巴细胞百分比(%) CD4+ T淋巴细胞百分比(%) CD8+ T淋巴细胞百分比(%) CD4+/CD8+值 联合治疗组 53 治疗前 41.28±4.47 23.15±2.56 29.38±2.18 0.79±0.16 治疗后 54.92±7.251) 34.95±3.111) 23.42±3.051) 1.50±0.321) t值 11.659 21.326 11.574 14.448 P值 <0.001 <0.001 <0.001 <0.001 对照组 50 治疗前 40.92±4.53 22.97±2.74 30.52±2.33 0.75±0.19 治疗后 40.85±4.11 23.41±2.93 29.74±3.42 0.78±0.22 t值 0.081 0.776 1.333 0.730 P值 0.936 0.440 0.186 0.467 注:1)与同期对照组比较,P<0.05。 表 3 两组患者肝功能及AFP水平比较
Table 3. Comparison of liver function and AFP levels between the two groups of patients
组别 例数 ALT(U/L) AST(U/L) Alb(g/L) AFP(ng/mL) 联合治疗组 53 治疗前 141.84±15.33 156.28±25.63 15.96±3.75 369.48±57.06 治疗后 55.63±10.761) 67.50±13.951) 38.69±7.301) 97.68±21.781) t值 33.510 22.149 20.164 32.398 P值 <0.001 <0.001 <0.001 <0.001 对照组 50 治疗前 143.52±16.46 159.84±28.79 16.15±2.97 379.65±65.34 治疗后 94.52±10.21 108.57±15.62 29.85±4.16 174.53±30.69 t值 17.888 11.068 18.952 20.092 P值 <0.001 <0.001 <0.001 <0.001 注:1)与同期对照组比较,P<0.05。 -
[1] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [2] WANG T, WANG CY, LIU JY, et al. Effectiveness and safety of ultrasound-guided ablation in treatment of primary liver cancer in dangerous areas[J]. J Clin Hepatol, 2021, 37( 7): 1594- 1598. DOI: 10.3969/j.issn.1001-5256.2021.07.023.王婷, 王春妍, 刘建勇, 等. 超声引导下局部消融治疗危险区域原发性肝癌的效果及安全性分析[J]. 临床肝胆病杂志, 2021, 37( 7): 1594- 1598. DOI: 10.3969/j.issn.1001-5256.2021.07.023. [3] MAO B, MA JD, DUAN SB, et al. Preoperative classification of primary and metastatic liver cancer via machine learning-based ultrasound radiomics[J]. Eur Radiol, 2021, 31( 7): 4576- 4586. DOI: 10.1007/s00330-020-07562-6. [4] ZHAI HG, XIAO ZH, YANG SL. Effect of CT-guided cryoablation with Argon-helium cryoablation on hepatic blood flow changes in primary liver cancer[J]. Chin J CT MRI, 2021, 19( 9): 96- 99, 110. DOI: 10.3969/j.issn.1672-5131.2021.09.030.翟焕阁, 肖正红, 杨双林. CT引导下氩氦刀冷冻消融术辅助治疗原发性肝癌对肿瘤组织血流变化的影响[J]. 中国CT和MRI杂志, 2021, 19( 9): 96- 99, 110. DOI: 10.3969/j.issn.1672-5131.2021.09.030. [5] LI X, MA LN, CHENG XZ, et al. Effect and safety of chemotherapy-based PD-1 inhibitors in the treatment of advanced lung adenocarcinoma[J]. J Pract Med, 2021, 37( 3): 365- 368. DOI: 10.3969/j.issn.1006-5725.2021.03.018.李兴, 马丽娜, 程小珍, 等. 基于化疗的PD-1抑制剂在晚期肺腺癌治疗中效果及安全性分析[J]. 实用医学杂志, 2021, 37( 3): 365- 368. DOI: 10.3969/j.issn.1006-5725.2021.03.018. [6] WANG Y, LUO D, LEI M, et al. Effect of silencing PD-1 gene on AAV8-mediated T cell immune response in HBV-infected rats[J]. Chin J Nosocomiology, 2021, 31( 17): 2584- 2588.王燕, 罗丹, 雷敏, 等. 沉默PD-1基因对AAV8介导的HBV感染大鼠T细胞免疫应答的影响[J]. 中华医院感染学杂志, 2021, 31( 17): 2584- 2588. [7] WEYKAMP F, HOEGEN P, REGNERY S, et al. Long-term clinical results of MR-guided stereotactic body radiotherapy of liver metastases[J]. Cancers(Basel), 2023, 15( 10): 2786. DOI: 10.3390/cancers15102786. [8] XU HC, WANG FL, XIE LH. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024.许华晨, 王凤玲, 谢林虎. 中晚期原发性肝细胞癌的临床治疗现状与展望[J]. 长春中医药大学学报, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024. [9] LIU Y, ZHENG JX, HAO JL, et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019[J]. Cancer Med, 2022, 11( 5): 1310- 1323. DOI: 10.1002/cam4.4551. [10] LUO J, LYU CH, YANG YP. Clinical efficacy and safety of percutaneous cryoablation combined with percutaneous ethanol injection in elderly patients with hepatocellular carcinoma aged 70 years or older[J]. J Clin Hepatol, 2022, 38( 2): 365- 371. DOI: 10.3969/j.issn.1001-5256.2022.02.021.罗婧, 吕采红, 杨永平. 经皮冷冻消融联合无水酒精注射治疗70岁以上老年肝细胞癌患者的效果及安全性分析[J]. 临床肝胆病杂志, 2022, 38( 2): 365- 371. DOI: 10.3969/j.issn.1001-5256.2022.02.021. [11] JIN ZQ, LIU YX, LIANG M, et al. Comparison of efficacy and safety between cryoballoon ablation and radiofrequency catheter ablation in the treatment of paroxysmal atrial fibrillation[J]. Clin J Med Offic, 2021, 49( 10): 1079- 1082. DOI: 10.16680/j.1671-3826.2021.10.05.金志清, 刘艳霞, 梁明, 等. 冷冻球囊消融术与射频导管消融术治疗阵发性心房颤动疗效及安全性比较[J]. 临床军医杂志, 2021, 49( 10): 1079- 1082. DOI: 10.16680/j.1671-3826.2021.10.05. [12] MA JB, WANG FM, ZHANG WQ, et al. Percutaneous cryoablation for the treatment of liver cancer at special sites: An assessment of efficacy and safety[J]. Quant Imaging Med Surg, 2019, 9( 12): 1948- 1957. DOI: 10.21037/qims.2019.11.12. [13] DONNE R, LUJAMBIO A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma[J]. Hepatology, 2023, 77( 5): 1773- 1796. DOI: 10.1002/hep.32740. [14] MERELLI B, MASSI D, CATTANEO L, et al. Targeting the PD1/PD-L1 axis in melanoma: Biological rationale, clinical challenges and opportunities[J]. Crit Rev Oncol Hematol, 2014, 89( 1): 140- 165. DOI: 10.1016/j.critrevonc.2013.08.002. [15] HEINRICH S, CRAIG AJ, MA LC, et al. Understanding tumour cell heterogeneity and its implication for immunotherapy in liver cancer using single-cell analysis[J]. J Hepatol, 2021, 74( 3): 700- 715. DOI: 10.1016/j.jhep.2020.11.036. [16] FREEMAN GJ, LONG AJ, IWAI Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation[J]. J Exp Med, 2000, 192( 7): 1027- 1034. DOI: 10.1084/jem.192.7.1027. [17] TOPALIAN SL, HODI FS, BRAHMER JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. N Engl J Med, 2012, 366( 26): 2443- 2454. DOI: 10.1056/nejmoa1200690. [18] FINN RS, ZHU AX. Evolution of systemic therapy for hepatocellular carcinoma[J]. Hepatology, 2021, 73( Suppl 1): 150- 157. DOI: 10.1002/hep.31306. [19] CHEN EB, YI J, JIANG J, et al. Identification and validation of a fatty acid metabolism-related lncRNA signature as a predictor for prognosis and immunotherapy in patients with liver cancer[J]. BMC Cancer, 2022, 22( 1): 1037. DOI: 10.1186/s12885-022-10122-4. [20] LAI FT. Clinical efficacy and safety of carrizumab in the treatment of advanced primary liver cancer[J]. Shanxi Med J, 2021, 50( 15): 2269- 2272.赖奉庭. 卡瑞利珠单抗治疗晚期原发性肝癌临床效果及安全性研究[J]. 山西医药杂志, 2021, 50( 15): 2269- 2272. [21] LEE YY, LUO SC, LEE CH, et al. Optimizing tumor-associated antigen-stimulated autologous dendritic cell and cytokine-induced killer cell coculture to enhance cytotoxicity for cancer immunotherapy in manufacturing[J]. BMC Immunol, 2023, 24( 1): 14. DOI: 10.1186/s12865-023-00552-5. -



 PDF下载 ( 883 KB)
PDF下载 ( 883 KB)

 下载:
下载: