不同来源的细胞外囊泡在肝细胞癌发生进展中的作用
DOI: 10.12449/JCH240630
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:史婷婷负责论文撰写与修改;张润兵、伍杨、张亚妮负责论文修改及审阅;朱玲玲、高春、江晶晶负责数据收集;张久聪、郑晓凤等负责拟定写作思路,指导撰写文章并最后定稿。
Role of extracellular vesicles of different origins in the development and progression of hepatocellular carcinoma
-
摘要: 肝细胞癌(HCC)是最常见的原发性肝癌类型,也是癌症相关死亡的第三大原因,严重威胁人体健康,成为目前亟待解决的临床难题。细胞外囊泡(EV)是一种含有多种成分的膜囊泡,在HCC的发生和进展中发挥着重要作用,本文通过总结不同来源的EV对HCC的影响,分析EV对HCC的作用机制,以期为HCC的诊断及治疗提供新视角。Abstract: Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and the third leading cause of cancer-related deaths, and it is a serious threat to human health and has become a clinical problem that needs to be solved urgently. Extracellular vesicles (EV) are membrane vesicles containing multiple components and play an important role in the development and progression of HCC. This article summarizes the effect of EVs of different origins on HCC and analyzes the mechanism of action of EV on HCC, so as to provide new perspectives for the diagnosis and treatment of HCC.
-
Key words:
- Carcinoma, Hepatocellular /
- Extracellular Vesicles /
- Diagnosis
-
-
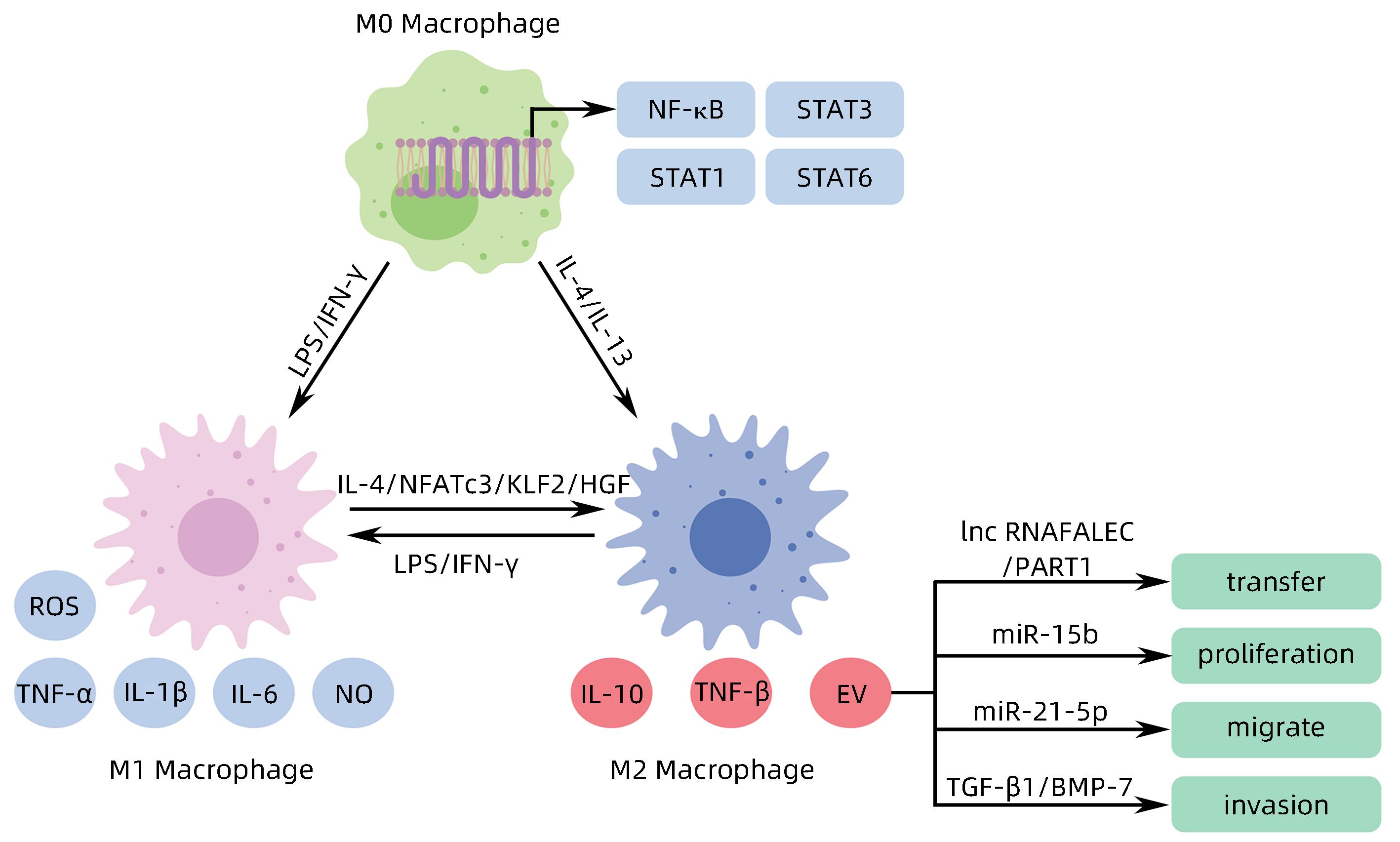
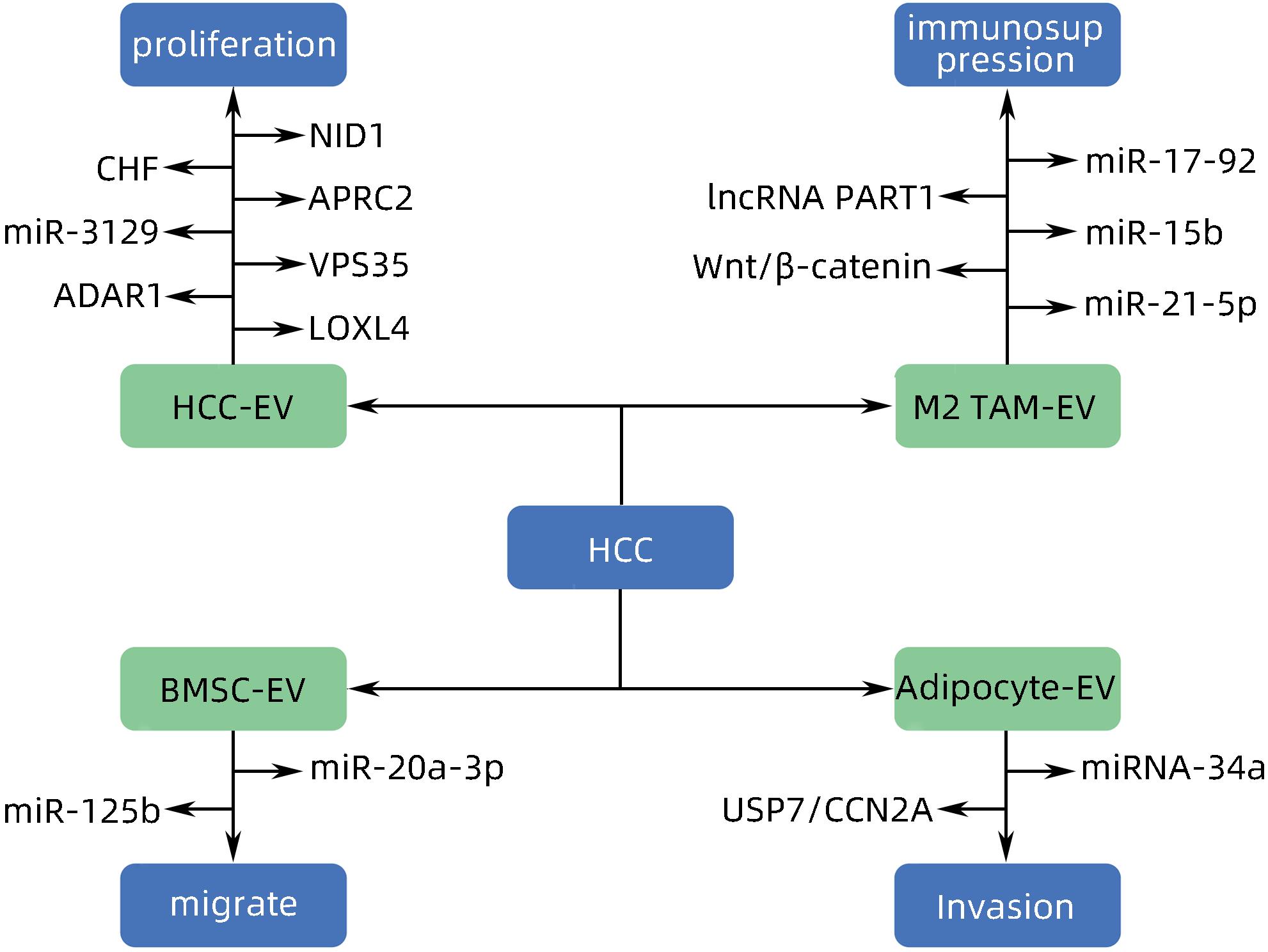
[1] WANG H, YU L, HUANG P, et al. Tumor-associated exosomes are involved in hepatocellular carcinoma tumorigenesis, diagnosis, and treatment[J]. J Clin Transl Hepatol, 2022, 10( 3): 496- 508. DOI: 10.14218/JCTH.2021.00425. [2] FORNER A, REIG M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2018, 391( 10127): 1301- 1314. DOI: 10.1016/s0140-6736(18)30010-2. [3] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380( 15): 1450- 1462. DOI: 10.1056/nejmra1713263. [4] HADE MD, SUIRE CN, SUO ZC. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine[J]. Cells, 2021, 10( 8): 1959. DOI: 10.3390/cells10081959. [5] HADE MD, SUIRE CN, MOSSELL J, et al. Extracellular vesicles: Emerging frontiers in wound healing[J]. Med Res Rev, 2022, 42( 6): 2102- 2125. DOI: 10.1002/med.21918. [6] MITANI F, LIN JY, SAKAMOTO T, et al. Asteltoxin inhibits extracellular vesicle production through AMPK/mTOR-mediated activation of lysosome function[J]. Sci Rep, 2022, 12( 1): 6674. DOI: 10.1038/s41598-022-10692-0. [7] WILLMS E, CABAÑAS C, MÄGER I, et al. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression[J]. Front Immunol, 2018, 9: 738. DOI: 10.3389/fimmu.2018.00738. [8] SINHA D, ROY S, SAHA P, et al. Trends in research on exosomes in cancer progression and anticancer therapy[J]. Cancers, 2021, 13( 2): 326. DOI: 10.3390/cancers13020326. [9] PAPADAKOS SP, DEDES N, PERGARIS A, et al. Exosomes in the treatment of pancreatic cancer: A moonshot to PDAC treatment?[J]. Int J Mol Sci, 2022, 23( 7): 3620. DOI: 10.3390/ijms23073620. [10] ZHAO L, PEI RF, DING YR, et al. LOXL4 shuttled by tumor cells-derived extracellular vesicles promotes immune escape in hepatocellular carcinoma by activating the STAT1/PD-L1 axis[J]. J Immunother, 2024, 47( 2): 64- 76. DOI: 10.1097/CJI.0000000000000496. [11] MAO XW, ZHOU LY, TEY SK, et al. Tumour extracellular vesicle-derived Complement Factor H promotes tumorigenesis and metastasis by inhibiting complement-dependent cytotoxicity of tumour cells[J]. J Extracell Vesicles, 2020, 10( 1): e12031. DOI: 10.1002/jev2.12031. [12] YANG Y, MAO FF, GUO L, et al. Tumor cells derived-extracellular vesicles transfer miR-3129 to promote hepatocellular carcinoma metastasis by targeting TXNIP[J]. Dig Liver Dis, 2021, 53( 4): 474- 485. DOI: 10.1016/j.dld.2021.01.003. [13] SHIBATA C, OTSUKA M, SHIMIZU T, et al. Extracellular vesicle-mediated RNA editing may underlie the heterogeneity and spread of hepatocellular carcinoma in human tissue and in vitro[J]. Oncol Rep, 2023, 50( 5): 194. DOI: 10.3892/or.2023.8631. [14] MAO XW, TEY SK, YEUNG CLS, et al. Nidogen 1-enriched extracellular vesicles facilitate extrahepatic metastasis of liver cancer by activating pulmonary fibroblasts to secrete tumor necrosis factor receptor 1[J]. Adv Sci, 2020, 7( 21): 2002157. DOI: 10.1002/advs.202002157. [15] MEI PR, TEY SK, WONG SWK, et al. Actin-related protein 2/3 complex subunit 2-enriched extracellular vesicles drive liver cancer metastasis[J]. Hepatol Int, 2022, 16( 3): 603- 613. DOI: 10.1007/s12072-022-10338-3. [16] LIU BHM, TEY SK, MAO XW, et al. TPI1-reduced extracellular vesicles mediated by Rab20 downregulation promotes aerobic glycolysis to drive hepatocarcinogenesis[J]. J Extracell Vesicles, 2021, 10( 10): e12135. DOI: 10.1002/jev2.12135. [17] TAN WC, ZHANG JX, LIU LX, et al. Hsp90 inhibitor STA9090 induced VPS35 related extracellular vesicle release and metastasis in hepatocellular carcinoma[J]. Transl Oncol, 2022, 26: 101502. DOI: 10.1016/j.tranon.2022.101502. [18] SICA A, MANTOVANI A. Macrophage plasticity and polarization: in vivo veritas[J]. J Clin Invest, 2012, 122( 3): 787- 795. DOI: 10.1172/JCI59643. [19] PELLO OM, PIZZOL MD, MIROLO M, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology[J]. Blood, 2012, 119( 2): 411- 421. DOI: 10.1182/blood-2011-02-339911. [20] ARORA S, DEV K, AGARWAL B, et al. Macrophages: Their role, activation and polarization in pulmonary diseases[J]. Immunobiology, 2018, 223( 4-5): 383- 396. DOI: 10.1016/j.imbio.2017.11.001. [21] DENARDO DG, RUFFELL B. Macrophages as regulators of tumour immunity and immunotherapy[J]. Nat Rev Immunol, 2019, 19( 6): 369- 382. DOI: 10.1038/s41577-019-0127-6. [22] BOUTILIER AJ, ELSAWA SF. Macrophage polarization states in the tumor microenvironment[J]. Int J Mol Sci, 2021, 22( 13): 6995. DOI: 10.3390/ijms22136995. [23] DENARDO DG, BARRETO JB, ANDREU P, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages[J]. Cancer Cell, 2009, 16( 2): 91- 102. DOI: 10.1016/j.ccr.2009.06.018. [24] ZHOU JY, CHE JH, XU L, et al. Tumor-derived extracellular vesicles containing long noncoding RNA PART1 exert oncogenic effect in hepatocellular carcinoma by polarizing macrophages into M2[J]. Dig Liver Dis, 2022, 54( 4): 543- 553. DOI: 10.1016/j.dld.2021.07.005. [25] LV SM, WANG JH, LI L. Extracellular vesicular lncRNA FAL1 promotes hepatocellular carcinoma cell proliferation and invasion by inducing macrophage M2 polarization[J]. J Physiol Biochem, 2023, 79( 3): 669- 682. DOI: 10.1007/s13105-022-00922-4. [26] LI JJ, XUE JC, LING M, et al. MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway[J]. Cancer Lett, 2021, 497: 137- 153. DOI: 10.1016/j.canlet.2020.10.023. [27] NING JY, YE YN, BU DC, et al. Imbalance of TGF-β1/BMP-7 pathways induced by M2-polarized macrophages promotes hepatocellular carcinoma aggressiveness[J]. Mol Ther, 2021, 29( 6): 2067- 2087. DOI: 10.1016/j.ymthe.2021.02.016. [28] PU J, XU ZM, NIAN JH, et al. M2 macrophage-derived extracellular vesicles facilitate CD8+ T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway[J]. Cell Death Discov, 2021, 7( 1): 182. DOI: 10.1038/s41420-021-00556-3. [29] WANG XB, YE XX, CHEN YP, et al. Mechanism of M2 type macrophage-derived extracellular vesicles regulating PD-L1 expression via the MISP/IQGAP1 axis in hepatocellular carcinoma immunotherapy resistance[J]. Int Immunopharmacol, 2023, 124( Pt A): 110848. DOI: 10.1016/j.intimp.2023.110848. [30] ZHANG HY, DENG T, GE SH, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7[J]. Oncogene, 2019, 38( 15): 2844- 2859. DOI: 10.1038/s41388-018-0619-z. [31] DENG L, WANG C, HE C, et al. Bone mesenchymal stem cells derived extracellular vesicles promote TRAIL-related apoptosis of hepatocellular carcinoma cells via the delivery of microRNA-20a-3p[J]. Cancer Biomark, 2021, 30( 2): 223- 235. DOI: 10.3233/CBM-201633. [32] DENG J, KE H. Overcoming the resistance of hepatocellular carcinoma to PD-1/PD-L1 inhibitor and the resultant immunosuppression by CD38 siRNA-loaded extracellular vesicles[J]. Oncoimmunology, 2023, 12( 1): 2152635. DOI: 10.1080/2162402X.2022.2152635. [33] BALDARI S, ROCCO GD, MAGENTA A, et al. Extracellular vesicles-encapsulated microRNA-125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation[J]. Cells, 2019, 8( 12): 1560. DOI: 10.3390/cells8121560. [34] CABIATI M, GIORGI ND, SALVADORI C, et al. Transcriptional level evaluation of osteopontin/miRNA-181a axis in hepatocellular carcinoma cell line-secreted extracellular vesicles[J]. Pathol Res Pract, 2022, 238: 154088. DOI: 10.1016/j.prp.2022.154088. -



 PDF下载 ( 878 KB)
PDF下载 ( 878 KB)


 下载:
下载: