经治慢性乙型肝炎患者低病毒血症发生率和影响因素的Meta分析
DOI: 10.12449/JCH240709
Incidence rate of low-level viremia and related influencing factors in treatment-experienced chronic hepatitis B patients: A Meta-analysis
-
摘要:
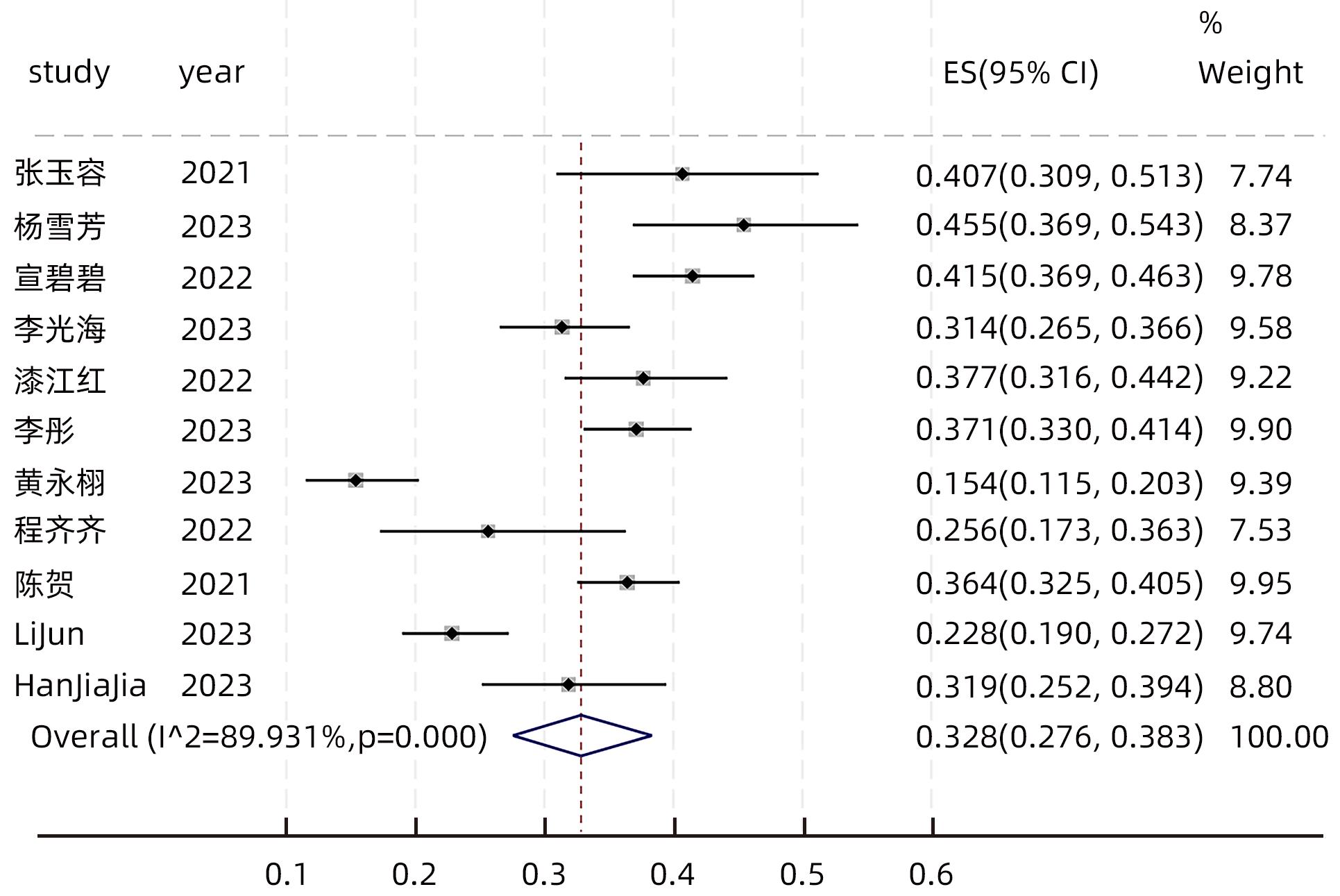
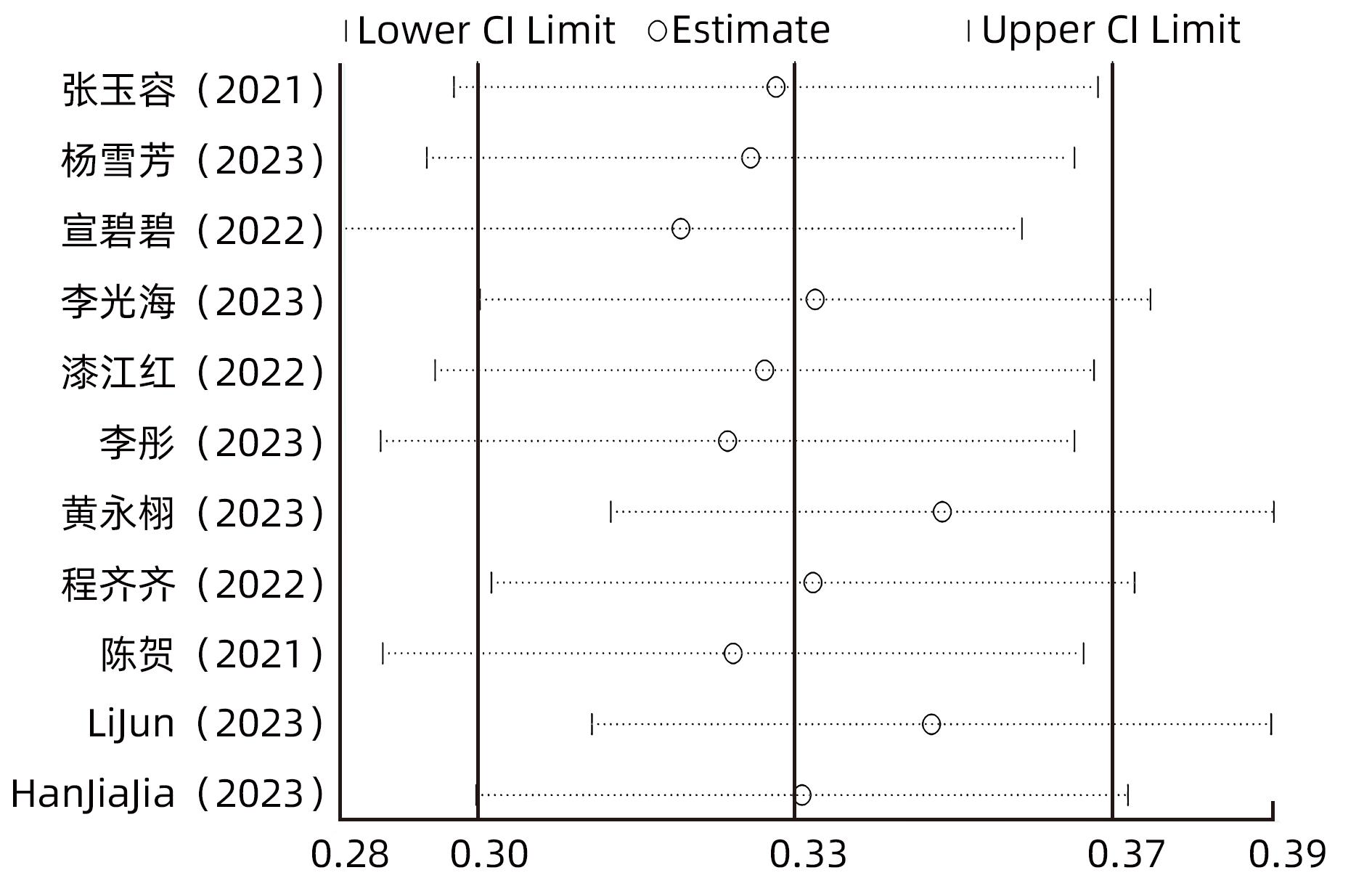
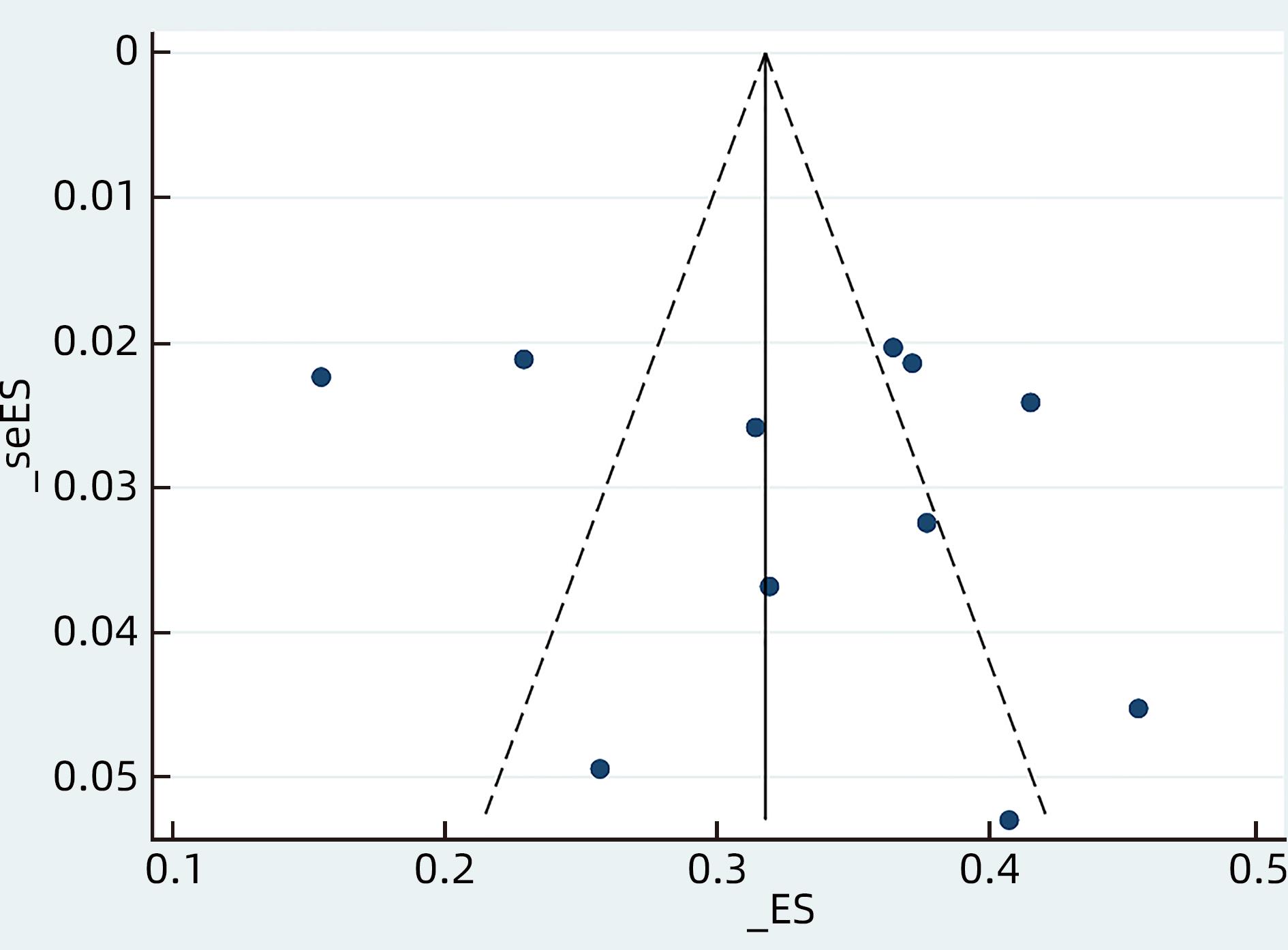
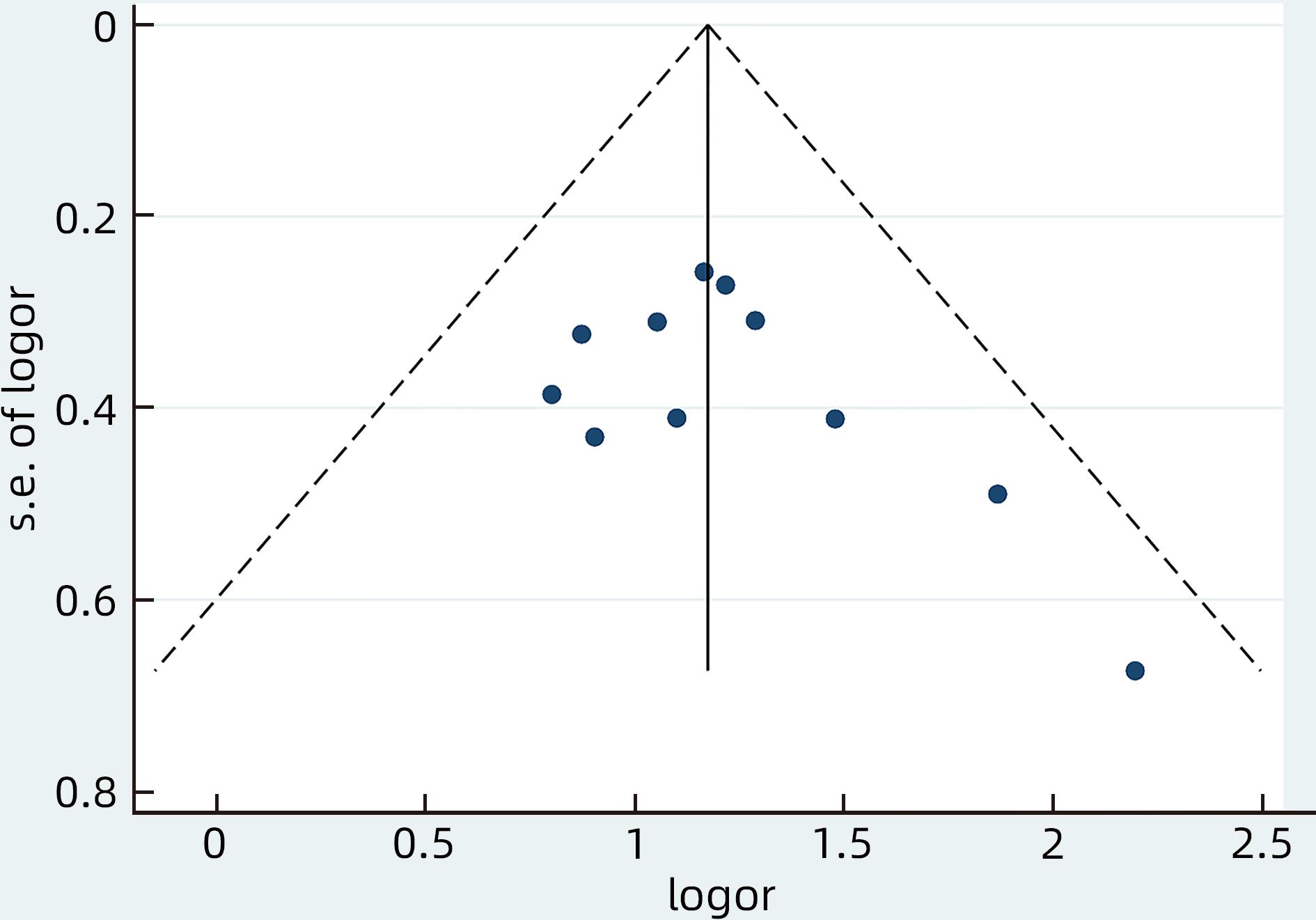
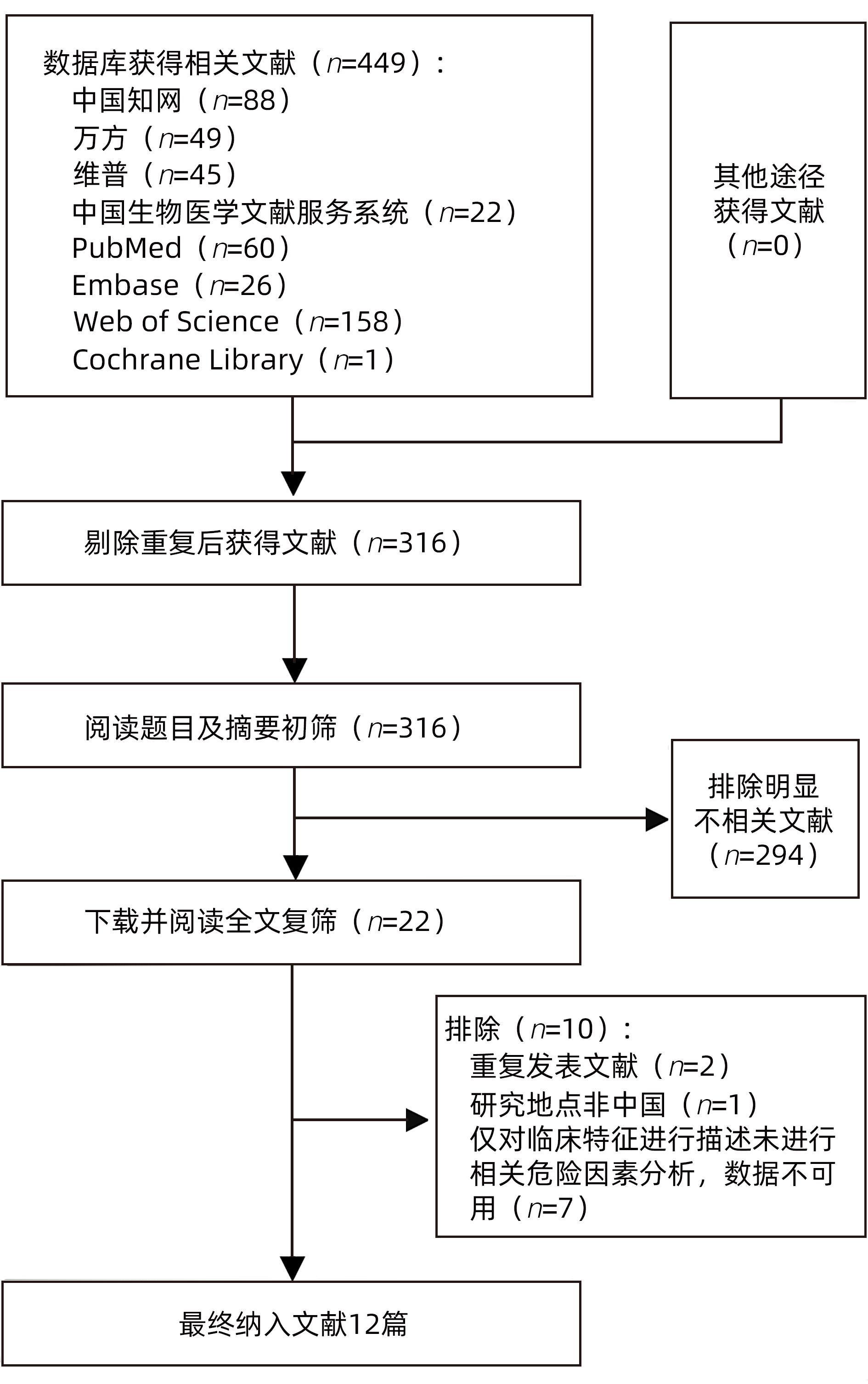
目的 系统评价慢性乙型肝炎(CHB)患者低病毒血症(LLV)的发生率及其影响因素,为临床有效干预和预防LLV的发生提供循证医学证据。 方法 本研究根据PRISMA指南完成,PROSPERO注册号:CRD42023455304。计算机检索中国知网、万方数据库、维普、中国生物医学文献服务系统、PubMed、Embase、Web of Science、Cochrane Library中有关CHB患者LLV发生及影响因素的观察性研究,检索时间为建库至2023年7月21日。应用Stata 16.0软件进行Meta分析。 结果 共纳入文献12篇,总样本量3 408例,包括LLV患者1 181例。Meta分析结果显示,经治CHB患者LLV发生率为32.8%(95%CI:27.6%~38.3%);HBsAg定量高(OR=2.107,95%CI:1.782~2.491,P<0.001)、HBeAg阳性(OR=3.258,95%CI:2.629~4.038,P<0.001)、高基线HBV DNA水平(OR=1.286,95%CI:1.157~1.430,P<0.001)及有恩替卡韦治疗史(OR=3.089,95%CI:1.880~5.074,P<0.001)是LLV发生的危险因素;抗病毒时间≥3年(OR=0.175,95%CI:0.093~0.331,P<0.001)和高基线ALT水平(OR=0.985,95%CI:0.978~0.992,P<0.001)是LLV的保护因素。敏感性分析显示效应值未发生明显变化,提示Meta分析结果相对稳定。纳入研究漏斗图基本对称,Egger’s检验和Begg’s检验结果提示纳入文献不存在明显发表偏倚。 结论 临床医生应根据LLV的影响因素,综合临床证据有效指导决策,降低远期临床风险,避免不良结局。 Abstract:Objective To systematically evaluate the incidence rate of low-level viremia (LLV) in chronic hepatitis B (CHB) patients and related influencing factors, and to provide evidence-based medicine evidence for effective intervention and prevention of LLV in clinical practice. Methods This study was conducted according to the PRISMA guideline, with a PROSPERO registration number of CRD42023455304. CNKI, Wanfang Data, VIP, SinoMed, PubMed, Embase, Web of Science, and the Cochrane library were searched for observational studies on LLV and related influencing factors in CHB patients published up to July 21, 2023. Stata 16.0 software was used to perform the meta-analysis. Results A total of 12 articles were included, with a total sample size of 3408 cases, among whom there were 1181 patients with LLV. The meta-analysis showed that the incidence rate of LLV was 32.8% (95% confidence interval [CI]: 27.6% — 38.3%) in treatment-experienced CHB patients. High HBsAg quantification (odds ratio [OR]=2.107, 95%CI: 1.782 — 2.491, P<0.001), positive HBeAg (OR=3.258, 95%CI: 2.629 — 4.038, P<0.001), high HBV DNA level at baseline (OR=1.286, 95%CI: 1.157 — 1.430, P<0.001), and history of entecavir treatment (OR=3.089, 95%CI: 1.880 — 5.074, P<0.001) were risk factors for LLV; duration of antiviral therapy ≥3 years (OR=0.175, 95%CI: 0.093 — 0.331, P<0.001) and high alanine aminotransferase level at baseline (OR=0.985, 95%CI: 0.978 — 0.992, P<0.001) were protective factors against LLV. The sensitivity analysis showed no significant change in effective value, suggesting that the results of the meta-analysis were relatively stable. The funnel plot of the studies included was basically symmetrical, and the results of the Egger’s test and the Begg’s test suggested that there was no obvious publication bias in the articles included. Conclusion Clinicians should guide decision making based on the influencing factors for LLV and related clinical evidence, so as to reduce long-term clinical risks and avoid adverse outcomes. -
Key words:
- Hepatitis B, Chronic /
- Low Level Viremia /
- Meta-Analysis
-
表 1 纳入文献基本特征及质量评价结果
Table 1. Basic characteristics and quality evaluation results
纳入文献 年份 研究类型 研究地点 地理区域 LLV(例) 总样本量(例) 影响因素 质量评价 危险 保护 评分 分级 张玉容[15] 2021 横断面 福建 南方 35 86 ①② ⑭ 6 中 杨雪芳等[16] 2023 横断面 云南 南方 55 121 ①③④ 6 中 宣碧碧等[17] 2022 横断面 山东 北方 173 417 ① ⑭⑮ 9 高 李光海等[18] 2023 横断面 海南 南方 101 322 ①⑥⑦ ⑬⑯ 5 中 漆江红[19] 2022 横断面 甘肃 北方 84 223 ①③⑥ 5 中 李彤等[20] 2023 横断面 甘肃 北方 189 509 ①③⑥⑧⑨ ⑪⑫ 7 中 黄永栩等[21] 2023 横断面 广东 南方 40 260 ①③⑦ 7 中 程齐齐等[22] 2022 横断面 江西 南方 20 78 ③⑥ ⑪ 6 中 陈贺等[23] 2021 横断面 江苏 南方 204 560 ①③⑥ 5 中 Lu等[24] 2022 病例对照 广东 南方 139 278 ①⑥ 5 中 Li等[25] 2023 队列 江苏 南方 90 394 ①⑤⑩ 7 高 Han等[26] 2023 横断面 上海 南方 51 160 ①⑧ ⑭ 7 中 注:①HBeAg阳性;②既往或目前使用非一线NAs类抗病毒药物;③基线HBV DNA水平高;④使用二线抗病毒药物;⑤HBV DNA水平≥1.0×108 IU/mL;⑥HBsAg定量高;⑦治疗期间依从性差;⑧ETV治疗史;⑨高HBeAg水平;⑩抗-HBc水平<3 log10 IU/mL;⑪基线ALT水平高;⑫治疗中HBV DNA水平下降幅度大;⑬治疗期间依从性好;⑭抗病毒治疗时间≥3年;⑮2年≤抗病毒治疗时间<3年;⑯基线HBV DNA水平低。 表 2 亚组分析结果
Table 2. Subgroup analysis results
表 3 影响因素的Meta分析结果
Table 3. Results of meta-analysis of influencing factors
影响因素 文献篇数 异质性 效应模型 合并效应量 P值 I2值 P值 OR(95%CI) Z值 HBeAg阳性 11[15-21,23-26] 0% 0.600 固定 3.258(2.629~4.038) 10.790 <0.001 基线HBV DNA水平高 6[16,19-23] 20.3% 0.285 固定 1.286(1.157~1.430) 4.656 <0.001 HBsAg定量高 6[18-20,22-24] 36.3% 0.165 固定 2.107(1.782~2.491) 8.728 <0.001 ETV治疗史 2[20,26] 10.2% 0.291 固定 3.089(1.880~5.074) 4.453 <0.001 基线ALT水平高 2[20,22] 48.2% 0.165 固定 0.985(0.978~0.992) -4.337 <0.001 抗病毒治疗时间≥3年 3[15,17,26] 0% 0.966 固定 0.175(0.093~0.331) -5.378 <0.001 -
[1] World Health Organization. Hepatitis B[EB/OL].( 2023-7-18)[ 2023-9-16]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b [2] WANG H, MEN PX, XIAO YF, et al. Hepatitis B infection in the general population of China: A systematic review and meta-analysis[J]. BMC Infect Dis, 2019, 19( 1): 811. DOI: 10.1186/s12879-019-4428-y. [3] LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63( 1): 284- 306. DOI: 10.1002/hep.28280. [4] CHEN CF, LEE WC, YANG HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma[J]. Gastroenterology, 2011, 141( 4): 1240- 1248. DOI: 10.1053/j.gastro.2011.06.036. [5] KIM GA, HAN S, CHOI GH, et al. Moderate levels of serum hepatitis B virus DNA are associated with the highest risk of hepatocellular carcinoma in chronic hepatitis B patients[J]. Aliment Pharmacol Ther, 2020, 51( 11): 1169- 1179. DOI: 10.1111/apt.15725. [6] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050. [7] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [8] YIN GQ, LI J, ZHONG B, et al. New therapeutic options for persistent low-level viremia in patients with chronic hepatitis B virus infection: Increase of entecavir dosage[J]. World J Gastroenterol, 2021, 27( 8): 666- 676. DOI: 10.3748/wjg.v27.i8.666. [9] LU FM, FENG B, ZHENG SJ, et al. Current status of the research on low-level viremia in chronic hepatitis B patients receiving nucleos(t)ide analogues[J]. J Clin Hepatol, 2021, 37( 6): 1268- 1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007.鲁凤民, 封波, 郑素军, 等. 核苷(酸)类似物经治的慢性乙型肝炎患者低病毒血症的研究现状[J]. 临床肝胆病杂志, 2021, 37( 6): 1268- 1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007. [10] SUN YM, WU XN, ZHOU JL, et al. Persistent low level of hepatitis B virus promotes fibrosis progression during therapy[J]. Clin Gastroenterol Hepatol, 2020, 18( 11): 2582- 2591. DOI: 10.1016/j.cgh.2020.03.001. [11] YANG J, CHOI WM, SHIM JH, et al. Low level of hepatitis B viremia compared with undetectable viremia increases the risk of hepatocellular carcinoma in patients with untreated compensated cirrhosis[J]. Am J Gastroenterol, 2023, 118( 6): 1010- 1018. DOI: 10.14309/ajg.0000000000002181. [12] SUN FR, LIU ZF, WANG BY. Correlation between low-level viremia and hepatitis B-related hepatocellular carcinoma and recurrence: A retrospective study[J]. BMC Cancer, 2021, 21( 1): 1103. DOI: 10.1186/s12885-021-08483-3. [13] LIU SN, GUO XF, WU DR, et al. Construction of clinical evidence grading system of TCM intervention based on evidence-based medicine principles[J]. Chin J Integr Tradit West Med, 2023, 43( 8): 911- 915.刘少南, 郭新峰, 吴大嵘, 等. 基于循证医学原则的中医干预类临床证据分级系统的构建[J]. 中国中西医结合杂志, 2023, 43( 8): 911- 915. [14] ZENG XT, LIU H, CHEN X, et al. Meta-analysis series IV: Quality evaluation tools for observational research[J]. Chin J Evid Based Cardiovasc Med, 2012, 4( 4): 297- 299. DOI: 10.3969/j.1674-4055.2012.04.004.曾宪涛, 刘慧, 陈曦, 等. Meta分析系列之四: 观察性研究的质量评价工具[J]. 中国循证心血管医学杂志, 2012, 4( 4): 297- 299. DOI: 10.3969/j.1674-4055.2012.04.004. [15] ZHANG YR. Profile of low level viremia in patients with chronic hepatitis B[D]. Fuzhou: Fujian Medical University, 2021.张玉容. 慢性乙型肝炎患者低病毒血症状况研究[D]. 福州: 福建医科大学, 2021. [16] YANG XF, XU Y, ZHANG BT, et al. Analysis of clinical characteristics and risk factors of low level viremia in patients with chronic hepatitis B after treatment[J]. Chin J Gastroenterol Hepatol, 2023, 32( 3): 276- 281. DOI: 10.3969/j.issn.1006-5709.2023.03.008.杨雪芳, 胥莹, 张帮婷, 等. 经治慢性乙型肝炎患者低病毒血症的临床特征及危险因素分析[J]. 胃肠病学和肝病学杂志, 2023, 32( 3): 276- 281. DOI: 10.3969/j.issn.1006-5709.2023.03.008. [17] XUAN BB, XU YH, DU ZC, et al. Influencing factors for low-level viremia in patients with chronic hepatitis B or hepatitis B liver cirrhosis and its association with the progression of liver inflammation and liver fibrosis[J]. J Clin Hepatol, 2022, 38( 10): 2252- 2259. DOI: 10.3969/j.issn.1001-5256.2022.10.011.宣碧碧, 徐永红, 杜忠彩, 等. 慢性乙型肝炎和乙型肝炎肝硬化患者发生低病毒血症的影响因素及其与肝脏炎症、肝纤维化进展的关系[J]. 临床肝胆病杂志, 2022, 38( 10): 2252- 2259. DOI: 10.3969/j.issn.1001-5256.2022.10.011. [18] LI GH, SUN L. Risk factors of low level viremia in chronic hepatitis B treated with nucleosides(acids)[J]. Med Health, 2023( 2): 165- 168.李光海, 孙龙. 核苷(酸)类药物治疗慢性乙型肝炎低病毒血症危险因素分析[J]. 医药卫生, 2023( 2): 165- 168. [19] QI JH. Analysis of influencing factors of hypoviremia in patients with chronic hepatitis B and liver cirrhosis[J]. J Front Med, 2022, 12( 35): 52- 54. DOI: 10.3969/j.issn.2095-1752.2022.35.015.漆江红. 慢性乙肝及肝硬化患者低病毒血症的影响因素分析[J]. 医药前沿, 2022, 12( 35): 52- 54. DOI: 10.3969/j.issn.2095-1752.2022.35.015. [20] LI T, KONG Y, LIU YY, et al. Demographic characteristics and associated influencing factors in treated patients with chronic hepatitis B with hypoviremia: A single-center retrospective cross-sectional study[J]. Chin J Hepatol, 2023, 31( 1): 42- 48. DOI: 10.3760/cma.j.cn501113-20220121-00039.李彤, 孔银, 刘元元, 等. 经治慢性乙型肝炎低病毒血症患者人群特征及其相关影响因素: 一项单中心横断面回顾性研究[J]. 中华肝脏病杂志, 2023, 31( 1): 42- 48. DOI: 10.3760/cma.j.cn501113-20220121-00039. [21] HUANG YX, CHEN C, BAO ZH, et al. Influencing factors of low-level viremia in patients with chronic hepatitis B treated with Entecavir[J]. Chin Hepatol, 2023, 28( 3): 320- 324. DOI: 10.3969/j.issn.1008-1704.2023.03.015.黄永栩, 陈超, 保紫红, 等. 恩替卡韦治疗的慢性乙型肝炎患者低病毒血症的影响因素[J]. 肝脏, 2023, 28( 3): 320- 324. DOI: 10.3969/j.issn.1008-1704.2023.03.015. [22] CHENG QQ, YANG LX, CAI TP, et al. Influencing factors for low-level viremia and their dynamic changes in patients with chronic hepatitis B treated with nucleos(t)ide analogues for the first time[J]. J Clin Hepatol, 2022, 38( 12): 2716- 2722. DOI: 10.3969/j.issn.1001-5256.2022.12.008.程齐齐, 杨丽霞, 蔡天盼, 等. 核苷(酸)类似物初治的慢性乙型肝炎患者发生低病毒血症的影响因素及其动态变化分析[J]. 临床肝胆病杂志, 2022, 38( 12): 2716- 2722. DOI: 10.3969/j.issn.1001-5256.2022.12.008. [23] CHEN H, FU JJ, LI L, et al. Influencing factors for low-level viremia in chronic hepatitis B patients treated with long-term entecavir antiviral therapy[J]. J Clin Hepatol, 2021, 37( 3): 556- 559. DOI: 10.3969/j.issn.1001-5256.2021.03.011.陈贺, 傅涓涓, 李丽, 等. 长期恩替卡韦经治慢性乙型肝炎患者低病毒血症的相关影响因素[J]. 临床肝胆病杂志, 2021, 37( 3): 556- 559. DOI: 10.3969/j.issn.1001-5256.2021.03.011. [24] LU JH, ZHANG CN, HE PY, et al. Risk factors for very low-level viremia in patients with chronic hepatitis B virus infection: A single-center retrospective study[J]. Liver Res, 2022, 6( 1): 39- 44. DOI: 10.1016/j.livres.2022.02.001. [25] LI J, DONG XQ, CAO LH, et al. Factors associated with persistent positive in HBV DNA level in patients with chronic Hepatitis B receiving entecavir treatment[J]. Front Cell Infect Microbiol, 2023, 13: 1151899. DOI: 10.3389/fcimb.2023.1151899. [26] HAN JJ, GUO YF, ZHANG XY, et al. Prevalence and associated factors of low-level viremia in chronic hepatitis B patients after long-term therapy with nucleos(t)ide analogs[J]. Turk J Gastroenterol, 2023, 34( 1): 53- 61. DOI: 10.5152/tjg.2023.21978. [27] LEE SB, JEONG J, PARK JH, et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir[J]. Clin Mol Hepatol, 2020, 26( 3): 364- 375. DOI: 10.3350/cmh.2020.0012. [28] AGARWAL K, BRUNETTO M, SETO WK, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection[J]. J Hepatol, 2018, 68( 4): 672- 681. DOI: 10.1016/j.jhep.2017.11.039. [29] PAVLOVIC V, YANG L, CHAN HLY, et al. Peginterferon alfa-2a(40 kD) stopping rules in chronic hepatitis B: A systematic review and meta-analysis of individual participant data[J]. Antivir Ther, 2019, 24( 2): 133- 140. DOI: 10.3851/IMP3304. [30] LU JH, YANG L, YE LH, et al. Clinical significance of HBsAg quantitative detection in the diagnosis and treatment of patients with chronic hepatitis B[J]. Mod J Integr Tradit Chin West Med, 2020, 29( 33): 3674- 3678. DOI: 10.3969/j.issn.1008-8849.2020.33.007.卢建华, 杨莉, 叶立红, 等. HBsAg定量检测在慢性乙型病毒性肝炎患者诊疗中的临床意义[J]. 现代中西医结合杂志, 2020, 29( 33): 3674- 3678. DOI: 10.3969/j.issn.1008-8849.2020.33.007. [31] HOOFNAGLE JH, DI BISCEGLIE AM. Serologic diagnosis of acute and chronic viral hepatitis[J]. Semin Liver Dis, 1991, 11( 2): 73- 83. DOI: 10.1055/s-2008-1040426. [32] ZHANG XJ, WU R, HUANG W, et al. Clinical study of hepatitis B virus RNA in different HBeAg states in chronic hepatitis B[J]. Chin J Health Lab Technol, 2023, 33( 3): 257- 260.张晓晶, 武瑞, 黄伟, 等. 乙型肝炎病毒RNA在慢性乙型病毒性肝炎不同HBeAg状态下的临床研究[J]. 中国卫生检验杂志, 2023, 33( 3): 257- 260. [33] LEE WM, KING WC, JANSSEN HLA, et al. Hepatitis B e antigen loss in adults and children with chronic hepatitis B living in North America: A prospective cohort study[J]. J Viral Hepat, 2021, 28( 11): 1526- 1538. DOI: 10.1111/jvh.13591. [34] LAU GKK, WANG FS. Uncover the immune biomarkers underlying hepatitis B e antigen(HBeAg) seroconversion: A need for more translational study[J]. J Hepatol, 2012, 56( 4): 753- 755. DOI: 10.1016/j.jhep.2011.12.006. [35] WANG CT, ZHANG YF, SUN BH, et al. Models for predicting hepatitis B e antigen seroconversion in response to interferon-α in chronic hepatitis B patients[J]. World J Gastroenterol, 2015, 21( 18): 5668- 5676. DOI: 10.3748/wjg.v21.i18.5668. [36] GENG MF, LI YX, GAO FY, et al. A scoring model predicts hepatitis B e antigen seroconversion in chronic hepatitis B patients treated with nucleos(t)ide analogs: Real-world clinical practice[J]. Int J Infect Dis, 2017, 62: 18- 25. DOI: 10.1016/j.ijid.2017.06.016. [37] HUDU SA, NIAZLIN MT, NORDIN SA, et al. Quantitative hepatitis B e antigen: A better predictor of hepatitis B virus DNA than quantitative hepatitis B surface antigen[J]. Clin Lab, 2018, 64( 4): 443- 449. DOI: 10.7754/Clin.Lab.2017.170916. [38] ZHANG C, LIU YQ, LI JW, et al. Dose-response relationship between qAnti-HBc and liver inflammation in chronic hepatitis B with normal or mildly elevated alanine transaminase based on liver biopsy[J]. J Med Virol, 2022, 94( 8): 3911- 3923. DOI: 10.1002/jmv.27779. [39] WANG XM, GAO XZ, WU RH, et al. Serum qAnti-HBc combined with ALT and HBsAg predicts significant hepatic inflammation in HBeAg-positive immune active patients[J]. J Gastroenterol Hepatol, 2022, 37( 9): 1806- 1814. DOI: 10.1111/jgh.15881. [40] LI J, MAO RC, LI XL, et al. A novel noninvasive index for the prediction of moderate to severe fibrosis in chronic hepatitis B patients[J]. Dig Liver Dis, 2018, 50( 5): 482- 489. DOI: 10.1016/j.dld.2017.12.028. [41] XIE YD, LI MH, OU XJ, et al. IP10 and anti-HBc can predict virological relapse and HBsAg loss in chronic hepatitis B patients after nucleos(t)ide analog discontinuation[J]. Dig Dis, 2023, 41( 6): 922- 931. DOI: 10.1159/000533515. [42] YU XG, DENG JK, HE XH, et al. Performance verification and clinical evaluation of HBV DNA high-sensitivity detection kit[J]. J Mol Diagn Ther, 2019, 11( 2): 111- 116. DOI: 10.3969/j.issn.1674-6929.2019.02.009.余学高, 邓间开, 何小洪, 等. HBV DNA高敏检测试剂盒的性能验证和临床应用评价[J]. 分子诊断与治疗杂志, 2019, 11( 2): 111- 116. DOI: 10.3969/j.issn.1674-6929.2019.02.009. [43] XIE L, LIU GW, GUO HJ. Analysis of the correlation between HBV DNA load level and hepatitis B hepatocellular carcinoma indicators and the effect on the outcome[J]. Chin J Integr Tradit West Med Liver Dis, 2023, 33( 10): 925- 929. DOI: 10.3969/j.issn.1005-0264.2023.010.014.谢露, 刘光伟, 郭会军. HBV DNA载量水平与乙型肝炎相关肝癌指标的相关性分析及其对预后的影响[J]. 中西医结合肝病杂志, 2023, 33( 10): 925- 929. DOI: 10.3969/j.issn.1005-0264.2023.010.014. [44] LI J, BI YH, HUANG YH, et al. Consistency and the value of high-sensitivity and conventional fluorescence quantitative PCR in different viral load in patients with chronic hepatitis B[J]. J Trop Med, 2023, 23( 2): 198- 202. DOI: 10.3969/j.issn.1672-3619.2023.02.013.李静, 毕燕华, 黄月华, 等. 高敏与普通荧光定量PCR技术在不同病毒载量慢乙肝患者HBV-DNA检测的一致性及应用价值[J]. 热带医学杂志, 2023, 23( 2): 198- 202. DOI: 10.3969/j.issn.1672-3619.2023.02.013. [45] LONG L, ZHANG Q, XIONG Y, et al. Application of high-sensitivity hepatitis B virus-DNA testing in the monitoring of antiviral therapy in patients with low-level viremia[J]. Guizhou Med J, 2022, 46( 8): 1182- 1183, 1186. DOI: 10.3969/j.issn.1000-744X.2022.08.002.龙黎, 张卿, 熊晏, 等. 高敏HBV-DNA检测在低病毒血症患者抗病毒治疗监测中的应用研究[J]. 贵州医药, 2022, 46( 8): 1182- 1183, 1186. DOI: 10.3969/j.issn.1000-744X.2022.08.002. [46] GISH RG, GIVEN BD, LAI CL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities[J]. Antiviral Res, 2015, 121: 47- 58. DOI: 10.1016/j.antiviral.2015.06.008. [47] SUN FL, XIA W, OUYANG YL. Research progress on detection methods for hepatitis B virus covalently closed circular DNA[J]. J Viral Hepat, 2023, 30( 5): 366- 373. DOI: 10.1111/jvh.13817. [48] PARK HD. Current status of clinical application of point-of-care testing[J]. Arch Pathol Lab Med, 2021, 145( 2): 168- 175. DOI: 10.5858/arpa.2020-0112-RA. [49] TIAN Y, FAN ZH, XU L, et al. CRISPR/Cas13a-assisted rapid and portable HBV DNA detection for low-level viremia patients[J]. Emerg Microbes Infect, 2023, 12( 1): e2177088. DOI: 10.1080/22221751.2023.2177088. [50] LIU LP, WU XP, CAI TP, et al. Analysis of efficacy and factors influencing sequential combination therapy with tenofovir alafenamide fumarate after treatment with entecavir in chronic hepatitis B patients with low-level viremia[J]. Chin J Hepatol, 2023, 31( 2): 118- 125. DOI: 10.3760/cma.j.cn501113-20221019-00507.刘丽萍, 邬小萍, 蔡天盼, 等. 恩替卡韦经治后低病毒血症的慢性乙型肝炎患者序贯联合富马酸丙酚替诺福韦治疗的疗效及影响因素分析[J]. 中华肝脏病杂志, 2023, 31( 2): 118- 125. DOI: 10.3760/cma.j.cn501113-20221019-00507. [51] ISHIDO S, TAMAKI N, UCHIHARA N, et al. Switching from entecavir to tenofovir alafenamide for maintaining complete virological response in chronic hepatitis B[J]. JGH Open, 2023, 7( 8): 567- 571. DOI: 10.1002/jgh3.12950. [52] SANAI FM, ALJAWAD M, ALGHAMDI AS, et al. Long-term health and economic benefits of switching to tenofovir alafenamide versus continuing on entecavir in chronic hepatitis B patients with low-level viremia in Saudi Arabia[J]. Saudi J Gastroenterol, 2024, 30( 1): 23- 29. DOI: 10.4103/sjg.sjg_170_23. [53] CHOI J, KIM HJ, LEE J, et al. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: A Korean nationwide cohort study[J]. JAMA Oncol, 2019, 5( 1): 30- 36. DOI: 10.1001/jamaoncol.2018.4070. [54] DUSHEIKO G. Will we need novel combinations to cure HBV infection?[J]. Liver Int, 2020, 40( Suppl 1): 35- 42. DOI: 10.1111/liv.14371. [55] GROSSI G, VIGANÒ M, LOGLIO A, et al. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues(NUCs)[J]. Liver Int, 2017, 37( Suppl 1): 45- 51. DOI: 10.1111/liv.13291. [56] PAPATHEODORIDIS GV, IDILMAN R, DALEKOS GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B[J]. Hepatology, 2017, 66( 5): 1444- 1453. DOI: 10.1002/hep.29320. [57] ALLARD NL, MACLACHLAN JH, DEV A, et al. Adherence in chronic hepatitis B: Associations between medication possession ratio and adverse viral outcomes[J]. BMC Gastroenterol, 2020, 20( 1): 140. DOI: 10.1186/s12876-020-01219-w. [58] SHIN JW, JUNG SW, LEE SB, et al. Medication nonadherence increases hepatocellular carcinoma, cirrhotic complications, and mortality in chronic hepatitis B patients treated with entecavir[J]. Am J Gastroenterol, 2018, 113( 7): 998- 1008. DOI: 10.1038/s41395-018-0093-9. [59] TAO YC, WANG ML, ZHANG DM, et al. Optimal drug administration manner would rescue partial virological response in chronic hepatitis B patients with entecavir or tenofovir treatment[J]. J Viral Hepat, 2020, 27( 7): 731- 738. DOI: 10.1111/jvh.13275. [60] WANG CH, CHANG KK, LIN RC, et al. Consolidation period of 18 months no better at promoting off-treatment durability in HBeAg-positive chronic hepatitis B patients with tenofovir disoproxil fumarate treatment than a 12-month period: A prospective randomized cohort study[J]. Medicine(Baltimore), 2020, 99( 18): e19907. DOI: 10.1097/MD.0000000000019907. [61] BOGLIONE L, CUSATO J, CARITI G, et al. Role of HBsAg decline in patients with chronic hepatitis B HBeAg-negative and E genotype treated with pegylated-interferon[J]. Antiviral Res, 2016, 136: 32- 36. DOI: 10.1016/j.antiviral.2016.10.011. [62] HANSEN BE, BUSTER EH, STEYERBERG EW, et al. Prediction of the response to peg-interferon-alfa in patients with HBeAg positive chronic hepatitis B using decline of HBV DNA during treatment[J]. J Med Virol, 2010, 82( 7): 1135- 1142. DOI: 10.1002/jmv.21778. [63] GAO P, LUO YP, LI JF, et al. Effects of hepatitis B virus on Th17, Treg and Th17/Treg ratio in different alanine aminetransferase stages[J]. J Peking Univ Health Sci, 2022, 54( 2): 272- 277. DOI: 10.19723/j.issn.1671-167X.2022.02.012.高鹏, 雒艳萍, 李俊峰, 等. 乙型肝炎病毒在不同丙氨酸氨基转移酶状态下对Th17、Treg细胞及其比率的影响[J]. 北京大学学报(医学版), 2022, 54( 2): 272- 277. DOI: 10.19723/j.issn.1671-167X.2022.02.012. [64] LAN YT, WANG ZL, TIAN P, et al. Treg/Th17 imbalance and its clinical significance in patients with hepatitis B-associated liver cirrhosis[J]. Diagn Pathol, 2019, 14( 1): 114. DOI: 10.1186/s13000-019-0891-4. [65] LIU NQ, LIU B, ZHANG L, et al. Recovery of circulating CD56dim NK cells and the balance of Th17/Treg after nucleoside analog therapy in patients with chronic hepatitis B and low levels of HBsAg[J]. Int Immunopharmacol, 2018, 62: 59- 66. DOI: 10.1016/j.intimp.2018.06.043. -



 PDF下载 ( 1341 KB)
PDF下载 ( 1341 KB)

 下载:
下载: