慢性乙型肝炎合并代谢相关脂肪性肝病患者病毒学特征的分层分析
DOI: 10.12449/JCH240710
Virological features of chronic hepatitis B patients with metabolic associated fatty liver disease: A stratified analysis
-
摘要:
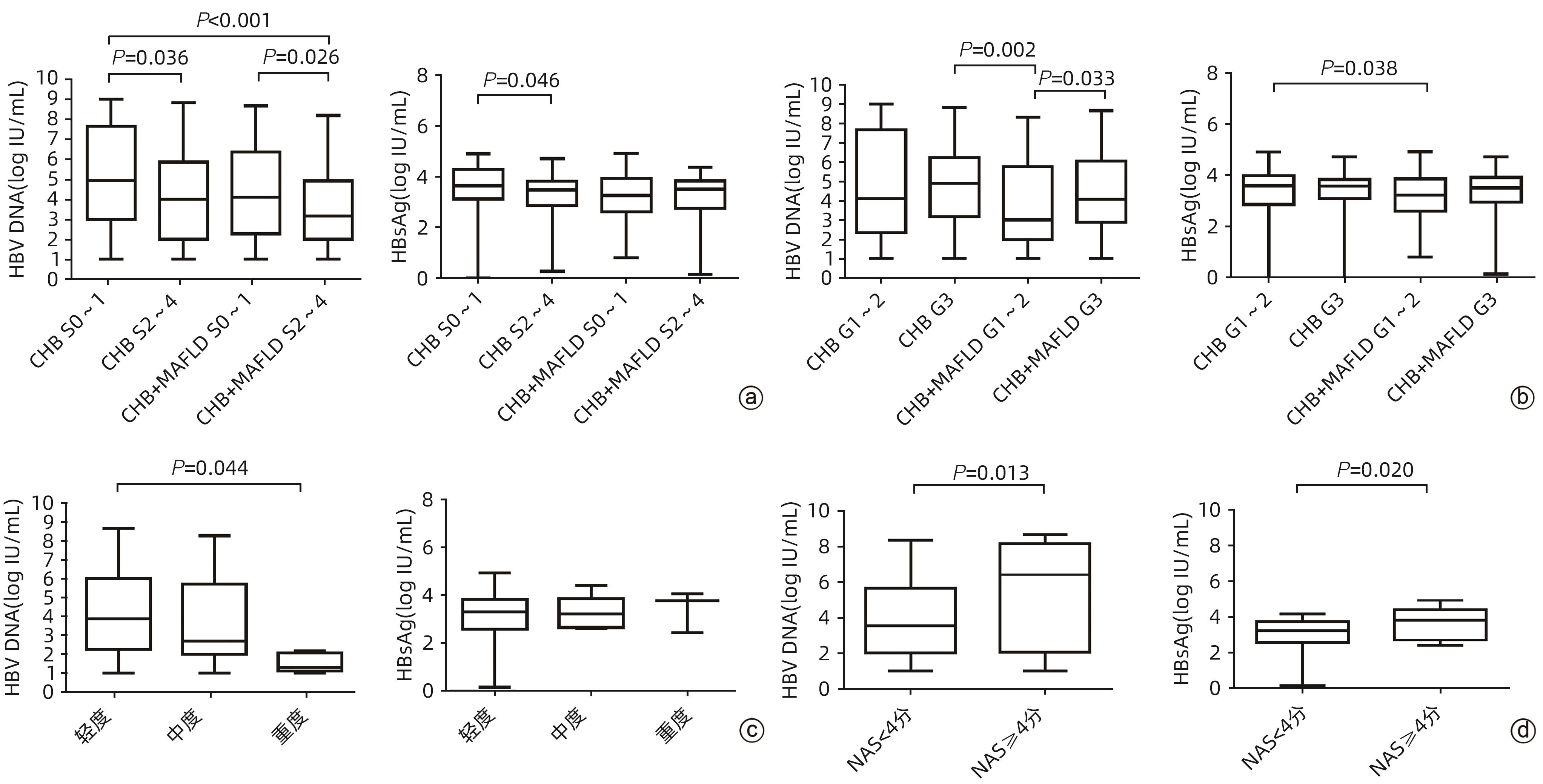
目的 分析不同分层的慢性乙型肝炎(CHB)合并代谢相关脂肪性肝病(MAFLD)患者的病毒学特征。 方法 回顾性选取2013年1月1日—2019年12月31日于首都医科大学附属北京佑安医院行经皮肝穿刺活检、未接受抗病毒治疗或接受治疗后停药6个月以上的CHB合并MAFLD患者131例和单纯CHB患者168例,比较两组患者的一般资料、血生化指标、病毒学指标;根据肝脏病理的炎症活动度(G)及肝纤维化分期(S)对两组患者进行分层,并依据肝脂肪变性程度及非酒精性脂肪性肝炎炎症活动度评分(NAS)对CHB合并MAFLD患者进一步分析,比较各组病毒学(血清HBV DNA和HBsAg水平)特征差异。计量资料两组间比较采用Wilcoxon检验;多组间比较及进一步两两比较均采用Kruskal-Wallis H检验。计数资料两组间比较采用χ2检验。 结果 CHB合并MAFLD患者中男性、高血压及2型糖尿病的比例,血生化指标甘油三酯、低密度脂蛋白胆固醇、载脂蛋白B、ALT、GGT、尿酸和空腹血糖均明显高于单纯CHB患者(P值均<0.05),而高密度脂蛋白胆固醇、载脂蛋白A1和HBV DNA水平均显著低于单纯CHB患者(P值均<0.05)。肝纤维化分期分层分析结果显示,单纯CHB组和CHB合并MAFLD组显著肝纤维化患者(S2~4)的HBV DNA水平均低于非显著肝纤维化患者(S0~1)(P值均<0.05);单纯CHB组显著肝纤维化患者(S2~4)的HBsAg水平明显低于非显著肝纤维化患者(S0~1)(P<0.05)。炎症活动度分层分析结果显示,CHB合并MAFLD组高炎症活动度患者(G3)的HBV DNA水平高于低炎症活动度患者(G1~2)(P<0.05);CHB合并MAFLD组低炎症活动度患者(G1~2)的HBsAg水平明显低于单纯CHB组低炎症活动度患者(P<0.05)。肝脂肪变性程度分层分析结果显示,HBV DNA水平随脂肪变性程度增加而逐渐降低,其中脂肪变性重度组HBV DNA水平明显低于轻度组(P<0.05),而HBsAg水平在不同肝脂肪变性程度组间无明显变化(P>0.05)。NAS分层分析结果显示,NAS≥4分组的HBV DNA和HBsAg水平均明显高于NAS<4分组(P值均<0.05)。 结论 CHB合并MAFLD患者具有明显的代谢指标和转氨酶水平异常,而病毒学指标在不同分层中表现出不同的特征。 Abstract:Objective To investigate the virological features of patients with chronic hepatitis B (CHB) and metabolic associated fatty liver disease (MAFLD) through a stratified analysis. Methods A retrospective analysis was performed for 131 patients with CHB and MAFLD and 168 patients with CHB alone who underwent percutaneous liver biopsy and did not receive antiviral therapy or withdrew from drugs for more than 6 months in Beijing YouAn Hospital, Capital Medical University, from January 1, 2013 to December 31, 2019. The two groups were compared in terms of general data, biochemical parameters, and virological parameters. The patients in the two groups were stratified according to liver inflammation grade (G) and liver fibrosis stage (S), and the patients with CHB and MAFLD were further analyzed based on the degree of hepatic steatosis and NAFLD activity score (NAS). Virological features (the serum levels of HBV DNA and HBV HBsAg) were compared between groups. The Wilcoxon test was used for comparison of continuous data between two groups, and the Kruskal-Wallis H test was used for comparison between multiple groups and further comparison between two groups; the chi-square test was used for comparison of categorical data between two groups. Results Compared with the CHB group, the CHB+MAFLD group had a significantly higher proportion of male patients, a significantly higher proportion of patients with hypertension or type 2 diabetes mellitus, and significantly higher levels of the blood biochemical parameters of triglyceride, low-density lipoprotein cholesterol, apolipoprotein B, alanine aminotransferase, gamma-glutamyl transpeptidase, uric acid, and fasting blood glucose (all P<0.05), as well as significantly lower levels of high-density lipoprotein cholesterol, apolipoprotein A1, and HBV DNA (all P<0.05). The stratified analysis based on liver fibrosis stage showed that for both the patients with CHB alone and those with CHB and MAFLD, the significant fibrosis (S2 — 4) group had a significantly lower level of HBV DNA than the non-significant fibrosis (S0 — 1) group (P<0.05), and for the patients with CHB alone, the significant fibrosis (S2 — 4) group had a significantly lower level of HBsAg than the non-significant fibrosis (S0 — 1) group (P<0.05). The stratified analysis based on inflammation grade showed that for the patients with CHB and MAFLD, the high inflammation grade (G3) group had a significantly higher level of HBV DNA than the low inflammation grade (G1 — 2) group (P<0.05), and in the low inflammation grade (G1 — 2) group, the patients with CHB and MAFLD had a significantly lower level of HBsAg than the patients with CHB alone (P<0.05). The stratified analysis based on the degree of hepatic steatosis showed that the level of HBV DNA gradually decreased with the increase in the degree of steatosis, and the severe steatosis group had a significantly lower level of HBV DNA than the mild group (P<0.05), while there was no significant difference in HBsAg level between the groups with different degrees of hepatic steatosis (P>0.05). The stratified analysis based on NAS score showed that the NAS≥4 group had significantly higher levels of HBV DNA and HBsAg than the NAS<4 group (both P<0.05). Conclusion Patients with CHB and MAFLD have significant abnormalities in metabolic markers and aminotransferases, while virological indicators show different features in stratified analyses based on various indicators. -
Key words:
- Hepatitis B, Chronic /
- Metabolic Associated Fatty Liver Disease /
- Virology
-
注: a,单纯CHB患者与CHB合并MAFLD患者不同肝纤维化分期的HBV DNA及HBsAg水平比较;b,单纯CHB患者与CHB合并MAFLD患者不同炎症活动度分级的HBV DNA及HBsAg水平比较;c,不同肝脂肪变性程度CHB合并MAFLD患者HBV DNA及HBsAg水平比较;d,不同NAS评分CHB合并MAFLD患者HBV DNA及HBsAg水平比较。
图 1 不同分层单纯CHB患者与CHB合并MAFLD患者的病毒学指标比较
Figure 1. Comparison of HBV virological indexes in different strata of patients with CHB and CHB combined with MAFLD
表 1 单纯CHB患者与CHB合并MAFLD患者一般资料及血生化和病毒学指标比较
Table 1. Baseline clinical characteristics, blood biochemical and virological indices in CHB and CHB combined with MAFLD
项目 单纯CHB组(n=168) CHB合并MAFLD组(n=131) 统计值 P值 一般资料 年龄(岁) 38(31~46) 39(35~48) Z=-1.956 0.051 性别[例(%)] χ2=9.263 <0.001 男 85(50.60) 94(71.76) 女 83(49.40) 37(28.24) BMI(kg/m2) 22.7(20.6~24.7) 26.3(24.8~28.8) Z=-9.099 <0.001 高血压[例(%)] 9(5.36) 21(16.03) χ2=9.289 0.002 2型糖尿病[例(%)] 6(3.57) 15(11.45) χ2=6.997 0.008 乙型肝炎家族史[例(%)] 53(31.55) 33(25.19) χ2=1.452 0.228 抗病毒治疗史[例(%)] 16(9.52) 2(1.53) χ2=8.320 0.004 血生化指标 甘油三酯(mmol/L) 0.95(0.73~1.36) 1.45(1.08~2.09) Z=-6.580 <0.001 胆固醇(mmol/L) 4.40(3.81~4.91) 4.70(4.02~5.27) Z=-1.789 0.074 高密度脂蛋白胆固醇(mmol/L) 1.27(1.00~1.53) 1.05(0.88~1.24) Z=-4.551 <0.001 低密度脂蛋白胆固醇(mmol/L) 2.58(2.07~2.96) 2.95(2.52~3.31) Z=-3.777 <0.001 脂蛋白(mmol/L) 70.0(34.0~184.4) 77.7(35.6~152.0) Z=-0.311 0.757 游离脂肪酸(mmol/L) 0.50(0.34~0.67) 0.47(0.36~0.65) Z=-0.066 0.948 载脂蛋白A1(g/L) 1.43(1.23~1.62) 1.34(1.18~1.48) Z=-2.645 0.008 载脂蛋白B(g/L) 0.75(0.66~0.89) 0.91(0.80~1.01) Z=-5.161 <0.001 ALT(U/L) 34.0(20.0~68.5) 46.0(29.0~85.3) Z=-3.194 0.001 AST(U/L) 30.5(22.8~47.3) 34.5(25.0~56.0) Z=-1.640 0.101 GGT(U/L) 25.0(15.0~58.0) 37.0(27.0~66.0) Z=-4.279 <0.001 尿酸(μmol/L) 305.0(251.0~348.0) 334.0(271.0~388.0) Z=-3.259 0.001 空腹葡萄糖(mmol/L) 4.92(4.61~5.33) 5.45(4.97~6.13) Z=-6.198 <0.001 病毒水平 HBV DNA(×103 IU/mL) 33.50(0.75~4 490.00) 4.67(0.10~798.00) Z=-2.013 0.044 HBsAg(×103 IU/mL) 3.30(1.02~8.19) 1.92(0.42~6.66) Z=-1.552 0.128 表 2 单纯CHB患者与CHB合并MAFLD患者不同肝纤维化分期及炎症活动度的分层分析
Table 2. Stratified analysis of different liver fibrosis stages and inflammatory activity in patients with CHB alone and patients with CHB combined with MAFLD
项目 单纯CHB组(n=168) CHB合并MAFLD组(n=131) 例数 HBV DNA (×103 IU/mL) HBsAg (×103 IU/mL) 例数 HBV DNA (×103 IU/mL) HBsAg (×103 IU/mL) 炎症活动度分级 低炎症活动度 86 13.45(0.28~45 200.00) 3.13(0.72~10.49) 61 1.05(0.10~561.00) 1.05(0.38~5.92) 高炎症活动度 82 80.90(1.78~1 682.50) 3.78(1.25~6.92) 70 11.85(0.96~998.50) 3.30(1.08~7.59) 肝纤维化分级 非显著肝纤维化 108 89.40(1.09~40 500.00) 4.18(1.33~16.48) 85 12.70(0.19~1 650.00) 2.00(0.53~8.51) 显著肝纤维化 60 10.35(0.10~530.25) 2.89(0.72~6.63) 46 1.49(0.10~73.73) 3.30(0.66~7.01) 表 3 CHB合并MAFLD患者不同肝脂肪变性程度及NAS评分的分层分析
Table 3. Stratified analysis of different degrees of hepatic steatosis and NAS scores in patients with CHB combined with MAFLD
项目 例数 HBV DNA(×103 IU/mL) HBsAg(×103 IU/mL) 脂肪变性程度 轻度 103 7.23(0.19~992.50) 2.00(0.38~6.62) 中度 24 0.50(0.10~233.93) 1.62(0.50~6.57) 重度 4 0.02(0.02~0.05) 5.87(3.07~8.68) NAS评分 NAS≥4分 28 2 590.00(0.14~130 250.00) 6.65(0.66~22.18) NAS<4分 103 3.45(0.10~426.50) 1.68(0.38~5.29) -
[1] JENG WJ, PAPATHEODORIDIS GV, LOK ASF. Hepatitis B[J]. Lancet, 2023, 401( 10381): 1039- 1052. DOI: 10.1016/S0140-6736(22)01468-4. [2] ESLAM M, NEWSOME PN, SARIN SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement[J]. J Hepatol, 2020, 73( 1): 202- 209. DOI: 10.1016/j.jhep.2020.03.039. [3] JIANG DX, CHEN C, LIU XX, et al. Concurrence and impact of hepatic steatosis on chronic hepatitis B patients: A systematic review and meta-analysis[J]. Ann Transl Med, 2021, 9( 23): 1718. DOI: 10.21037/atm-21-3052. [4] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Inf, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [5] HUANG SC, LIU CJ. Chronic hepatitis B with concurrent metabolic dysfunction-associated fatty liver disease: Challenges and perspectives[J]. Clin Mol Hepatol, 2023, 29( 2): 320- 331. DOI: 10.3350/cmh.2022.0422. [6] HUANG SC, SU TH, TSENG TC, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B[J]. Hepatol Int, 2023, 17( 5): 1139- 1149. DOI: 10.1007/s12072-023-10545-6. [7] WANG CC, KAO JH. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study[J]. Hepatology, 2017, 66( 2): 681. DOI: 10.1002/hep.29252. [8] PAIS R, RUSU E, ZILISTEANU D, et al. Prevalence of steatosis and insulin resistance in patients with chronic hepatitis B compared with chronic hepatitis C and non-alcoholic fatty liver disease[J]. Eur J Intern Med, 2015, 26( 1): 30- 36. DOI: 10.1016/j.ejim.2014.12.001. [9] HUANG JF, JING ML, WANG CY, et al. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study[J]. J Med Virol, 2020, 92( 8): 1191- 1197. DOI: 10.1002/jmv.25621. [10] LIU WH, LIU H, DING HG, et al. Clinical characteristics and prognosis of patients with chronic hepatitis B combined with metabolic associated fatty liver disease[J]. J Clin Hepatol, 2022, 38( 10): 2230- 2235. DOI: 10.3969/j.issn.1001-5256.2022.10.007.刘伟鸿, 刘晖, 丁惠国, 等. 慢性乙型肝炎合并代谢相关性脂肪性肝病的临床特征及预后影响因素分析[J]. 临床肝胆病杂志, 2022, 38( 10): 2230- 2235. DOI: 10.3969/j.issn.1001-5256.2022.10.007. [11] van KLEEF LA, CHOI HSJ, BROUWER WP, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B[J]. JHEP Rep, 2021, 3( 5): 100350. DOI: 10.1016/j.jhepr.2021.100350. [12] HAAM JH, LEE YK, SUH E, et al. Characteristics of urine organic acid metabolites in nonalcoholic fatty liver disease assessed using magnetic resonance imaging with elastography in Korean adults[J]. Diagnostics(Basel), 2022, 12( 5): 1199. DOI: 10.3390/diagnostics12051199. [13] ZHAO CC, WANG AP, LI LX, et al. Urine uric acid excretion is associated with nonalcoholic fatty liver disease in patients with type 2 diabetes[J]. J Diabetes Complications, 2016, 30( 6): 1074- 1080. DOI: 10.1016/j.jdiacomp.2016.04.017. [14] WANG RS, XUE FB, WANG LP, et al. Serum uric acid to creatinine ratio is associated with higher prevalence of NAFLD detected by FibroScan in the United States[J]. J Clin Lab Anal, 2022, 36( 8): e24590. DOI: 10.1002/jcla.24590. [15] HUI RWH, SETO WK, CHEUNG KS, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case-control study[J]. J Viral Hepat, 2018, 25( 1): 97- 104. DOI: 10.1111/jvh.12766. [16] WANG MM, WANG GS, SHEN F, et al. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors[J]. Dig Dis Sci, 2014, 59( 10): 2571- 2579. DOI: 10.1007/s10620-014-3180-9. [17] ZHENG Q, ZOU BY, WU YK, et al. Systematic review with meta-analysis: Prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B[J]. Aliment Pharmacol Ther, 2021, 54( 9): 1100- 1109. DOI: 10.1111/apt.16595. [18] YI ST, REN GH, ZHU Y, et al. Correlation analysis of hepatic steatosis and hepatitis B virus: A cross-sectional study[J]. Virol J, 2024, 21( 1): 22. DOI: 10.1186/s12985-023-02277-8. [19] LIU QC, MU MY, CHEN H, et al. Hepatocyte steatosis inhibits hepatitis B virus secretion via induction of endoplasmic reticulum stress[J]. Mol Cell Biochem, 2022, 477( 11): 2481- 2491. DOI: 10.1007/s11010-021-04143-z. [20] YOON S, JUNG J, KIM T, et al. Adiponectin, a downstream target gene of peroxisome proliferator-activated receptor γ, controls hepatitis B virus replication[J]. Virology, 2011, 409( 2): 290- 298. DOI: 10.1016/j.virol.2010.10.024. [21] YANG T, LI J. Chronic hepatitis B comorbid with nonalcoholic fatty liver disease: Contemporary insights and controversies[J]. J Clin Hepatol, 2023, 39( 8): 1797- 1804. DOI: 10.3969/j.issn.1001-5256.2023.08.005.阳韬, 李军. 慢性乙型肝炎合并非酒精性脂肪性肝病: 当前的认识与争议[J]. 临床肝胆病杂志, 2023, 39( 8): 1797- 1804. DOI: 10.3969/j.issn.1001-5256.2023.08.005. -



 PDF下载 ( 1033 KB)
PDF下载 ( 1033 KB)

 下载:
下载:


