细胞周期蛋白依赖性激酶1(CDK1)和极光激酶A(AURKA)在HBV相关肝细胞癌患者血清中的表达及意义
DOI: 10.12449/JCH240717
Expression and clinical significance of cell cycle protein-dependent kinase 1 and aurora kinase A in the serum of patients with hepatitis B virus-related hepatocellular carcinoma
-
摘要:
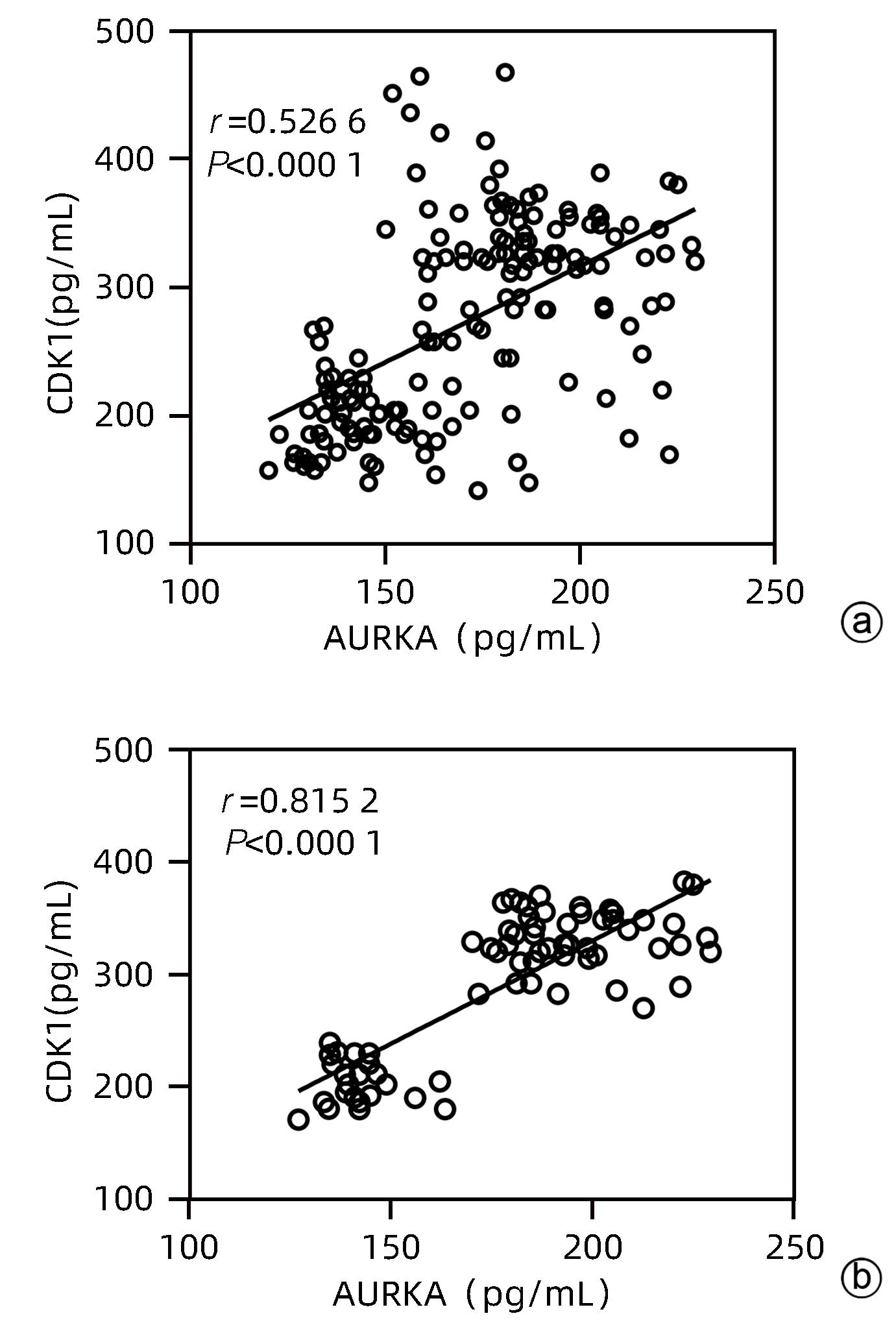
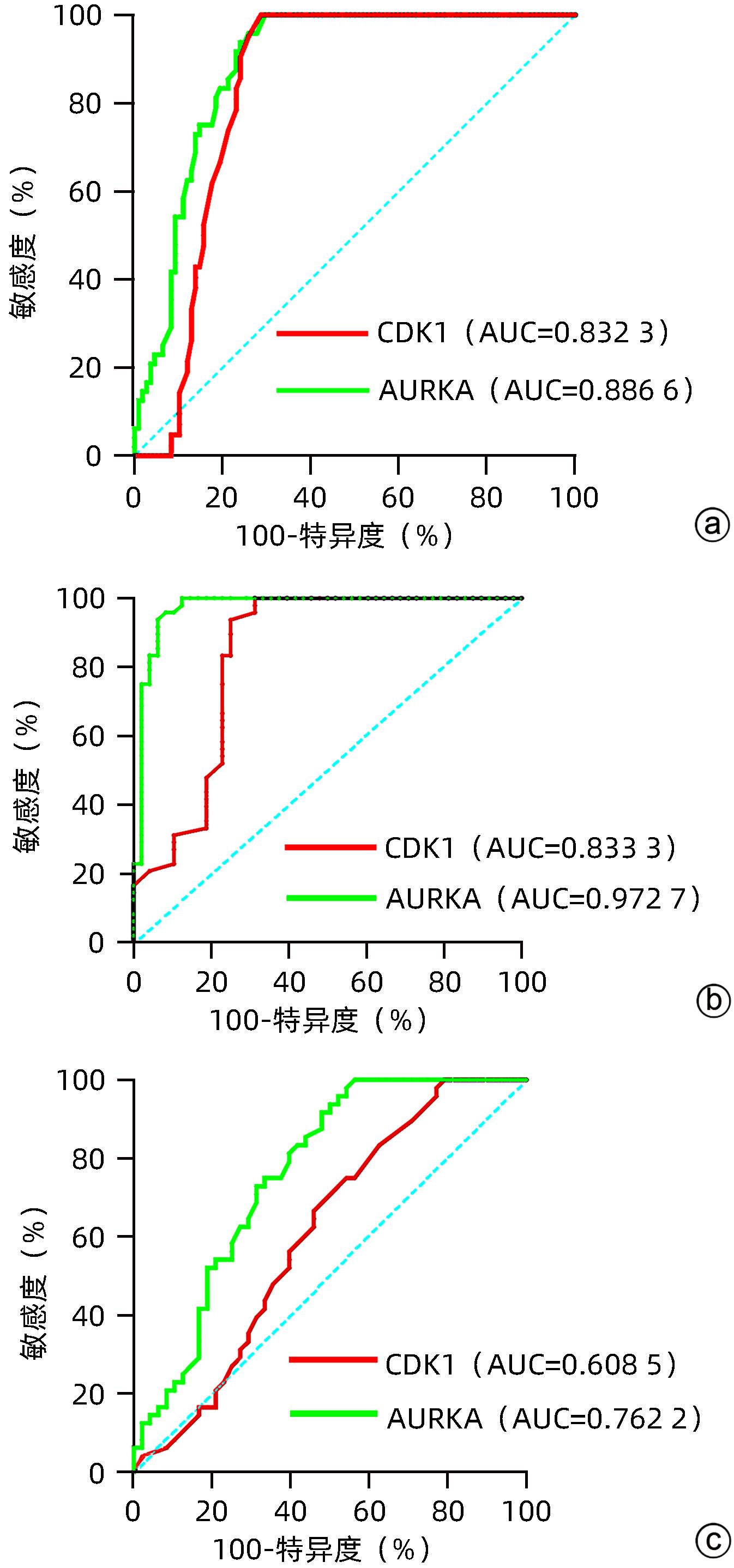
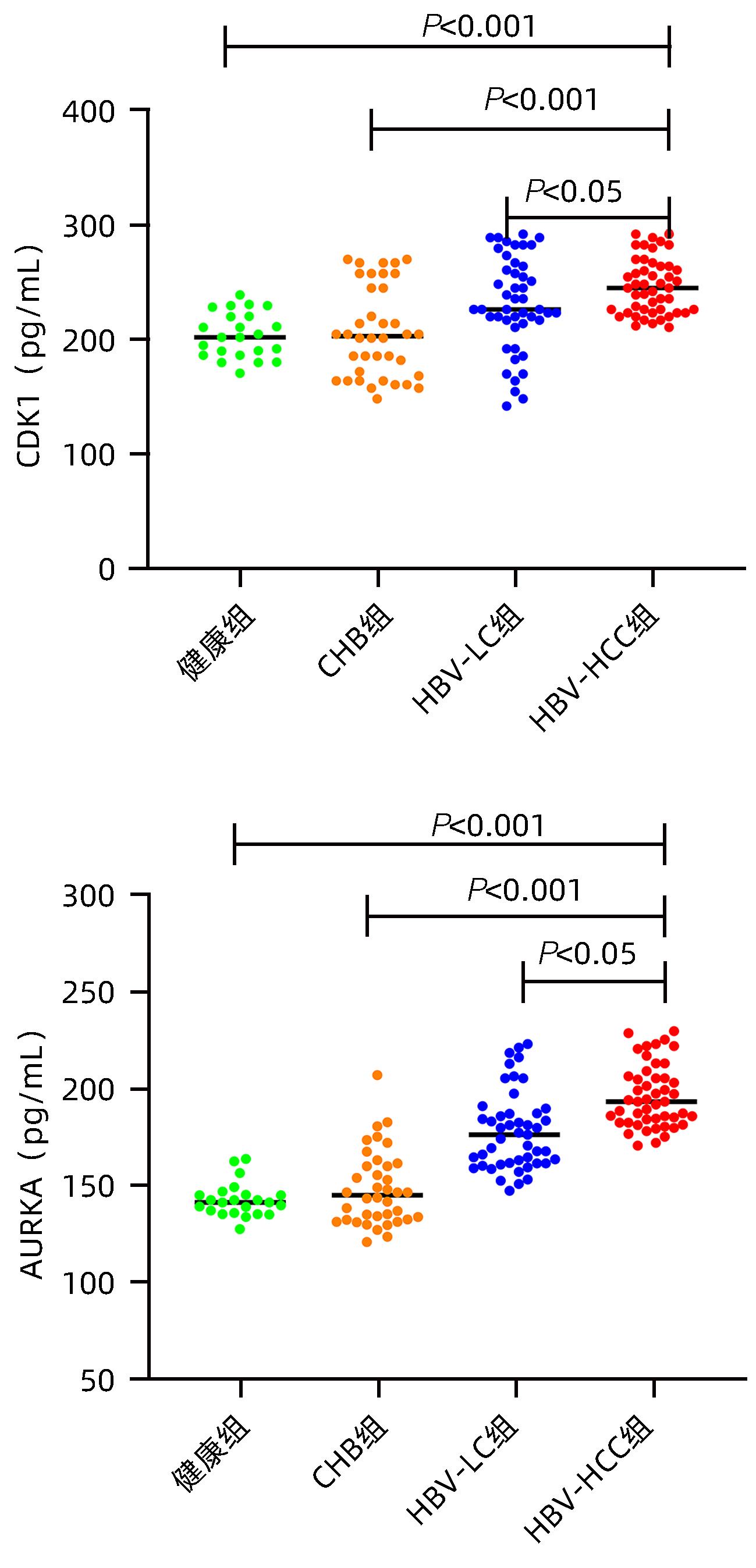
目的 探讨血清细胞周期蛋白依赖性激酶1(CDK1)和极光激酶A(AURKA)在HBV相关肝细胞癌 (HBV-HCC)患者中的诊断价值。 方法 收集2022年6月—2023年12月在甘肃省人民医院消化内科住院部的HBV-HCC患者、HBV相关肝硬化患者(HBV-LC)及慢性乙型肝炎患者(CHB)各50例,收集同期体检中心与病例组年龄、性别相匹配的健康人群50例,记录患者的年龄、性别、并发症以及入院后首次的血常规、肝功能、凝血等检验指标。ELISA法检测各组血清中CDK1和AURKA水平。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Bonferroni检验。计数资料组间比较采用χ2检验或Fisher确切概率法。CDK1与AURKA表达的关系采用Spearman相关分析;受试者工作特征曲线(ROC曲线)下面积(AUC)分析CDK1与AURKA对HBV-HCC的诊断价值。 结果 与对照组相比,HBV-HCC患者肝功能各项指标差异均有统计学意义(P值均<0.05);CHB组与HBV-HCC组Alb、Glb、DBil、AST、GGT、ALP水平比较差异均有统计学意义(P值均<0.05);HBV-LC组与HBV-HCC组Glb、AST、GGT水平比较差异均有统计学意义(P值均<0.05)。HBV-HCC组患者血清中CDK1及AURKA水平明显高于HBV-LC组、CHB组和对照组(P值均<0.05)。CDK1水平与AURKA在总研究人群和HBV-HCC患者中均存在显著的正相关性(r值分别为0.526 6、0.815 2,P值均<0.001)。以对照组为参照,CDK1诊断HBV-HCC的AUC为0.832 3,敏感度为92.86%,特异度为75%;AURKA诊断HCC的AUC为0.886 6,敏感度为95.80%,特异度为74%。以CHB组为参照,CDK1诊断HBV-HCC的AUC为0.833 3,敏感度为93.75%,特异度为75%;AURKA诊断HBV-HCC的AUC为0.972 7,敏感度为95.83%,特异度为91.67%。以HBV-LC组为参照,CDK1诊断HBV-HCC的AUC为0.608 5,敏感度为66.67%,特异度为54.17%;AURKA诊断HBV-HCC的AUC为0.762 2,敏感度为95.83%,特异度为47.92%。 结论 血清CDK1与AURKA水平随着HBV相关慢性肝病的进展而升高,二者对HBV相关HCC有一定的诊断价值。 -
关键词:
- 癌, 肝细胞 /
- 细胞周期蛋白质依赖激酶类 /
- 极光激酶A
Abstract:Objective To investigate the value of serum cell cycle protein-dependent kinase 1 (CDK1) and aurora kinase A (AURKA) in the diagnosis of patients with hepatitis B virus-related hepatocellular carcinoma (HBV-HCC). Methods A total of 50 HBV-HCC patients, 50 patients with hepatitis B virus-related liver cirrhosis (HBV-LC), and 50 chronic hepatitis B (CHB) patients who were hospitalized in Department of Gastroenterology, Gansu Provincial Hospital, from June 2022 to December 2023 were enrolled, and 50 healthy individuals, matched for age and sex, who received physical examination at Physical Examination Center during the same period of time were enrolled as control group. Related data were recorded for all patients, including age, sex, complications, and the results of routine blood test, liver function, and coagulation for the first time after admission. ELISA was used to measure the serum levels of CDK1 and AURKA. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups and the least significant difference Bonferroni test was used for further comparison between two groups; the chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups. The Spearman correlation analysis was used to investigate the correlation between CDK1 and AURKA, and the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to investigate the value of CDK1 and AURKA in the diagnosis of HBV-HCC. Results There were significant differences in liver function parameters between the HBV-HCC patients and the control group (all P<0.05); there were significant differences between the CHB group and the HBV-HCC group in albumin, Glb, direct bilirubin, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), and alkaline phosphatase (all P<0.05); there were significant differences between the HBV-LC group and the HBV-HCC group in Glb, AST, and GGT (all P<0.05). The HBV-HCC group had significantly higher serum levels of CDK1 and AURKA than the HBV-LC group, the CHB group, and the control group (all P<0.05). There was a significant positive correlation between CDK1 and AURKA in the overall study population and the HBV-HCC patients (r=0.526 6 and 0.815 2, P<0.001). With the control group as reference, CDK1 had an AUC of 0.832 3 in the diagnosis of HBV-HCC, with a sensitivity of 92.86% and a specificity of 75%, and AURKA had an AUC of 0.886 6 in the diagnosis of HCC, with a sensitivity of 95.80% and a specificity of 74%. With the CHB group as reference, CDK1 had an AUC of 0.833 3 in the diagnosis of HBV-HCC, with a sensitivity of 93.75% and a specificity of 75%, and AURKA had an AUC of 0.972 7 in the diagnosis of HBV-HCC, with a sensitivity of 95.83% and a specificity of 91.67%. With the HBV-LC group as reference, CDK1 had an AUC of 0.608 5 in the diagnosis of HBV-HCC, with a sensitivity of 66.67% and a specificity of 54.17%, and AURKA had an AUC of 0.762 2 in the diagnosis of HBV-HCC, with a sensitivity of 95.83% and a specificity of 47.92%. Conclusion The serum levels of CDK1 and AURKA increase with the progression of hepatitis B-associated chronic liver disease, and significant increases in serum CDK1 and AURKA have a certain value in the diagnosis of HBV-HCC. -
Key words:
- Carcinoma, Hepatocellular /
- Cyclin-Dependent Kinases /
- Aurora Kinase A
-
表 1 患者基本资料的比较
Table 1. Comparison of basic data of patients
组别 例数 男/女(例) 年龄(岁) 对照组 50 28/22 53.09±5.43 HBV-HCC组 50 34/16 56.04±2.70 HBV-LC组 50 28/22 55.83±3.58 CHB组 50 36/14 54.71±3.12 统计值 χ2=7.72 F=3.11 P值 0.52 0.07 表 2 各组肝功能指标的比较
Table 2. Comparison of liver function indexes in each group
指标 对照组(n=50) HBV-HCC组(n=50) HBV-LC组(n=50) CHB组(n=50) 统计值 P值 TP(g/L) 72.76±4.56 66.94±8.261) 68.68±8.20 70.57±9.02 F=3.25 0.024 Alb(g/L) 44.04±3.12 35.03±5.781) 33.64±6.24 40.68±5.562) F=25.30 <0.001 Glb(g/L) 28.72±3.10 31.93±4.861) 36.92±7.562) 27.99±4.752) F=21.17 <0.001 TBil(µmoL/L) 16.30(12.10~19.40) 28.27(19.12~46.54)1) 32.90(21.33~51.30) 26.14(15.50~36.28) H=20.58 <0.001 DBil(µmoL/L) 4.30(3.30~5.00) 9.30(5.81~21.85)1) 10.98(5.90~18.45) 4.61(3.40~9.70)2) H=35.64 <0.001 IBil(µmoL/L) 11.50(8.10~13.30) 17.10(11.80~23.26)1) 18.60(15.20~28.81) 14.22(11.57~30.38) H=15.02 0.002 AST(U/L) 27.00(19.80~29.00) 54.50(38.00~81.71)1) 35.00(24.00~35.00)2) 22.16(16.00~34.00)2) H=39.28 <0.001 ALT(U/L) 19.00(12.00~30.00) 39.11(25.33~65.00)1) 26.00(13.72~58.00) 25.00(13.00~70.00) H=11.90 0.008 GGT(U/L) 18.20(15.40~55.60) 68.00(40.70~150.82)1) 30.72(15.80~58.20)2) 18.33(13.09~40.06)2) H=36.25 <0.001 ALP(U/L) 71.00(54.00~87.00) 103.84(86.40~232.95)1) 81.00(58.00~114.00) 93.00(79.21~121.00)2) H=30.35 <0.001 注:TP,总蛋白;Glb,球蛋白。与对照组比较,1)P<0.05;与HBV-HCC组比较,2)P<0.05。 表 3 CDK1及AURKA与肝功能各项指标的相关性(rs )
Table 3. Correlation between CDK1、AURKA and liver function indexe (rs )
指标 对照组 CHB组 HBV-LC组 HBV-HCC组 CDK1 AURKA CDK1 AURKA CDK1 AURKA CDK1 AURKA TP 0.142 -0.249 -0.049 -0.058 0.009 -0.094 -0.3142) -0.2471) Alb 0.142 -0.079 -0.138 -0.133 -0.2961) -0.5762) -0.5572) -0.5702) Glb 0.215 -0.320 0.140 0.068 0.3162) 0.4852) 0.152 0.3442) TBil 0.054 0.250 0.221 0.158 0.167 0.3872) 0.4892) 0.3382) DBil 0.063 0.252 0.2561) 0.188 0.229 0.4782) 0.4952) 0.4062) IBil 0.029 0.212 0.184 0.146 0.177 0.2871) 0.4202) 0.2471) ALT 0.001 0.045 0.2731) 0.081 0.188 0.2631) 0.3342) 0.2931) AST 0.095 0.174 0.2621) 0.038 0.198 0.3682) 0.5782) 0.4782) GGT 0.063 0.305 0.169 0.118 0.063 0.081 0.4372) 0.3802) ALP 0.350 0.258 0.039 0.092 0.3332) 0.3452) 0.4492) 0.4432) 注:1)P<0.05;2)P<0.01。 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [2] CHEDID MF, KRUEL CRP, PINTO MA, et al. Hepatocellular carcinoma: Diagnosis and operative management[J]. Arq Bras Cir Dig, 2017, 30( 4): 272- 278. DOI: 10.1590/0102-6720201700040011. [3] CHEN DH, HONG M, ZHANG YL, et al. Expression and clinical significance of HBx and miR-122 in hepatitis B related hepatocellular carcinoma[J]. J Mol Diagn Ther, 2023, 15( 2): 240- 243, 248. DOI: 10.19930/j.cnki.jmdt.2023.02.029.陈东海, 洪玫, 张依琳, 等. 乙肝相关性肝细胞癌中HBx、miR-122表达及临床意义[J]. 分子诊断与治疗杂志, 2023, 15( 2): 240- 243, 248. DOI: 10.19930/j.cnki.jmdt.2023.02.029. [4] ZHU HZ, ZHOU WJ, WAN YF, et al. Downregulation of orosomucoid 2 acts as a prognostic factor associated with cancer-promoting pathways in liver cancer[J]. World J Gastroenterol, 2020, 26( 8): 804- 817. DOI: 10.3748/wjg.v26.i8.804. [5] HUANG YH, SRAMKOSKI RM, JACOBBERGER JW. The kinetics of G2 and M transitions regulated by B cyclins[J]. PLoS One, 2013, 8( 12): e80861. DOI: 10.1371/journal.pone.0080861. [6] ZOU YP, RUAN SY, JIN L, et al. CDK1, CCNB1, and CCNB2 are prognostic biomarkers and correlated with immune infiltration in hepatocellular carcinoma[J]. Med Sci Monit, 2020, 26: e925289. DOI: 10.12659/MSM.925289. [7] HANAHAN D, WEINBERG RA. Hallmarks of cancer: The next generation[J]. Cell, 2011, 144( 5): 646- 674. DOI: 10.1016/j.cell.2011.02.013. [8] JENG YM, PENG SY, LIN CY, et al. Overexpression and amplification of Aurora-A in hepatocellular carcinoma[J]. Clin Cancer Res, 2004, 10( 6): 2065- 2071. DOI: 10.1158/1078-0432.ccr-1057-03. [9] Bureau of Medical Administration, National Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China(2019 edition)[J]. J Clin Hepatol, 2020, 36( 2): 277- 292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36( 2): 277- 292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [10] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [11] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Inf, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01..中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [12] LI J, HAN X, YU XN, et al. Clinical applications of liquid biopsy as prognostic and predictive biomarkers in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA[J]. J Exp Clin Cancer Res, 2018, 37( 1): 213. DOI: 10.1186/s13046-018-0893-1. [13] MA CY, FU YL, ZHANG ZL, et al. The value of contrast-enhanced ultrasound in evaluating the efficacy of microwave ablation for hepatocellular carcinoma and predicting local recurrence[J]. Clin J Med Offic, 2023, 51( 8): 850- 853. DOI: 10.16680/j.1671-3826.2023.08.21.马春燕, 符叶柳, 张植兰, 等. 超声造影对肝细胞癌微波消融疗效评估及对局部复发预测价值研究[J]. 临床军医杂志, 2023, 51( 8): 850- 853. DOI: 10.16680/j.1671-3826.2023.08.21. [14] MALOV SI, MALOV IV, DVORNICHENKO VV, et al. Application of alpha-fetoprotein and osteopontin combination for early diagnosis of hepatocellular carcinoma associated with hepatitis C[J]. Klin Lab Diagn, 2019, 64( 10): 607- 612. DOI: 10.18821/0869-2084-2019-64-10-607-612. [15] YU XP, YANG RY, HE ZM, et al. Establishment and validation of nomogram of cancer specific survival of patients with hepatocellular carcinoma with negative alpha fetoprotein based on SEER Database[J]. J Jilin Univ(Med Edit), 2024, 50( 1): 188- 197. DOI: 10.13481/j.1671-587X.20240123.余孝鹏, 杨仁义, 贺佐梅, 等. 基于SEER数据库建立和验证甲胎蛋白阴性肝细胞癌患者癌症特异生存期的列线图[J]. 吉林大学学报(医学版), 2024, 50( 1): 188- 197. DOI: 10.13481/j.1671-587X.20240123. [16] PENG QZ, HAO JW, QIN Y, et al. Effect of DNA damage repair pathway mediated by AURKA on HepG2 of liver cancer cells[J]. Chongqing Med, 2021, 50( 1): 1- 7. DOI: 10.3969/j.issn.1671-8348.2021.01.001.彭期臻, 郝剑文, 秦宇, 等. AURKA介导DNA损伤修复通路对肝癌细胞HepG2的影响[J]. 重庆医学, 2021, 50( 1): 1- 7. DOI: 10.3969/j.issn.1671-8348.2021.01.001. [17] ZHANG L, HUANG Y, LING JJ, et al. Screening and function analysis of hub genes and pathways in hepatocellular carcinoma via bioinformatics approaches[J]. Cancer Biomark, 2018, 22( 3): 511- 521. DOI: 10.3233/CBM-171160. [18] BAO ZY, LU L, LIU XY, et al. Association between the functional polymorphism Ile31Phe in the AURKA gene and susceptibility of hepatocellular carcinoma in chronic hepatitis B virus carriers[J]. Oncotarget, 2017, 8( 33): 54904- 54912. DOI: 10.18632/oncotarget.18613. [19] LIU C, ZHU XX, JIA YQ, et al. Dasatinib inhibits proliferation of liver cancer cells, but activation of Akt/mTOR compromises dasatinib as a cancer drug[J]. Acta Biochim Biophys Sin, 2021, 53( 7): 823- 836. DOI: 10.1093/abbs/gmab061. [20] LI XC, XU WQ, KANG W, et al. Genomic analysis of liver cancer unveils novel driver genes and distinct prognostic features[J]. Theranostics, 2018, 8( 6): 1740- 1751. DOI: 10.7150/thno.22010. [21] WANG LL, JIN XH, CAI MY, et al. AGBL2 promotes cancer cell growth through IRGM-regulated autophagy and enhanced Aurora A activity in hepatocellular carcinoma[J]. Cancer Lett, 2018, 414: 71- 80. DOI: 10.1016/j.canlet.2017.11.003. [22] CHEN CL, SONG GY, XIANG J, et al. AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma[J]. Biochem Biophys Res Commun, 2017, 486( 2): 514- 520. DOI: 10.1016/j.bbrc.2017.03.075. [23] GOOS JACM, COUPE VMH, DIOSDADO B, et al. Aurora kinase A(AURKA) expression in colorectal cancer liver metastasis is associated with poor prognosis[J]. Br J Cancer, 2013, 109( 9): 2445- 2452. DOI: 10.1038/bjc.2013.608. [24] TANG AQ, GAO KY, CHU LL, et al. Aurora kinases: Novel therapy targets in cancers[J]. Oncotarget, 2017, 8( 14): 23937- 23954. DOI: 10.18632/oncotarget.14893. [25] ZHANG LJ, SHI H, JIANG ZY, et al. The mechanism of AURKA regulation of tumor cisplatin resistance[J]. Chem Life, 2023, 43( 8): 1268- 1276. DOI: 10.13488/j.smhx.20220609.张丽君, 石皓, 姜卓言, 等. AURKA调控肿瘤顺铂耐药机制[J]. 生命的化学, 2023, 43( 8): 1268- 1276. DOI: 10.13488/j.smhx.20220609. [26] WU CX, WANG XQ, CHOK SH, et al. Blocking CDK1/PDK1/β-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment bytargeting cancer stem cells in a preclinical model of hepatocellular carcinoma[J]. Theranostics, 2018, 8( 14): 3737- 3750. DOI: 10.7150/thno.25487. [27] TANG WW, CHEN ZY, ZHANG WL, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects[J]. Signal Transduct Target Ther, 2020, 5( 1): 87. DOI: 10.1038/s41392-020-0187-x. [28] YAN M, BAI RX, CHENG S, et al. Expression and significance of cyclin-dependent kinase 1 in human colorectal cancer[J]. Chin J Clin Dr, 2021, 49( 9): 1080- 1082. DOI: 10.3969/j.issn.2095-8552.2021.09.021.闫鸣, 白日星, 程石, 等. 细胞周期蛋白依赖性激酶1在人结直肠癌组织中的表达意义[J]. 中国临床医生杂志, 2021, 49( 9): 1080- 1082. DOI: 10.3969/j.issn.2095-8552.2021.09.021. [29] FANG XT, WU YY, YU TT, et al. Fusobacterium nucleatum promotes colorectal cancer by up-regulating Cdk1 through intestinal metabolite sodium butyrate[J]. Chin J Cell Biol, 2021, 43( 5): 979- 990. DOI: 10.11844/cjcb.2021.05.0009.方晓婷, 吴依依, 余婷婷, 等. 具核梭杆菌通过调节肠道代谢产物丁酸钠上调Cdk1促进结直肠癌发展[J]. 中国细胞生物学学报, 2021, 43( 5): 979- 990. DOI: 10.11844/cjcb.2021.05.0009. [30] NOZOE T, TAKAHASHI I, BABA H, et al. Relationship between intracellular localization of p34cdc2 protein and differentiation of esophageal squamous cell carcinoma[J]. J Cancer Res Clin Oncol, 2005, 131( 3): 179- 183. DOI: 10.1007/s00432-004-0607-2. [31] MA XL, HUANG SH, HUANG JA, et al. Expression and significance of CyclinB1 and CDK1 in gastric cancer[J]. Chin J Curr Adv Gen Surg, 2004, 7( 5): 272- 274. DOI: 10.3969/j.issn.1009-9905.2004.05.007.马晓丽, 黄淑红, 黄敬爱, 等. CyclinB1、CDK1在胃癌中的表达及其意义[J]. 中国现代普通外科进展, 2004, 7( 5): 272- 274. DOI: 10.3969/j.issn.1009-9905.2004.05.007. [32] SU Y, PAN SJ, LI ZQ, et al. Multiplex imaging and cellular target identification of kinase inhibitors via an affinity-based proteome profiling approach[J]. Sci Rep, 2015, 5: 7724. DOI: 10.1038/srep07724. [33] MALUMBRES M, BARBACID M. Cell cycle, CDKs and cancer: A changing paradigm[J]. Nat Rev Cancer, 2009, 9( 3): 153- 166. DOI: 10.1038/nrc2602. -



 PDF下载 ( 1048 KB)
PDF下载 ( 1048 KB)

 下载:
下载: