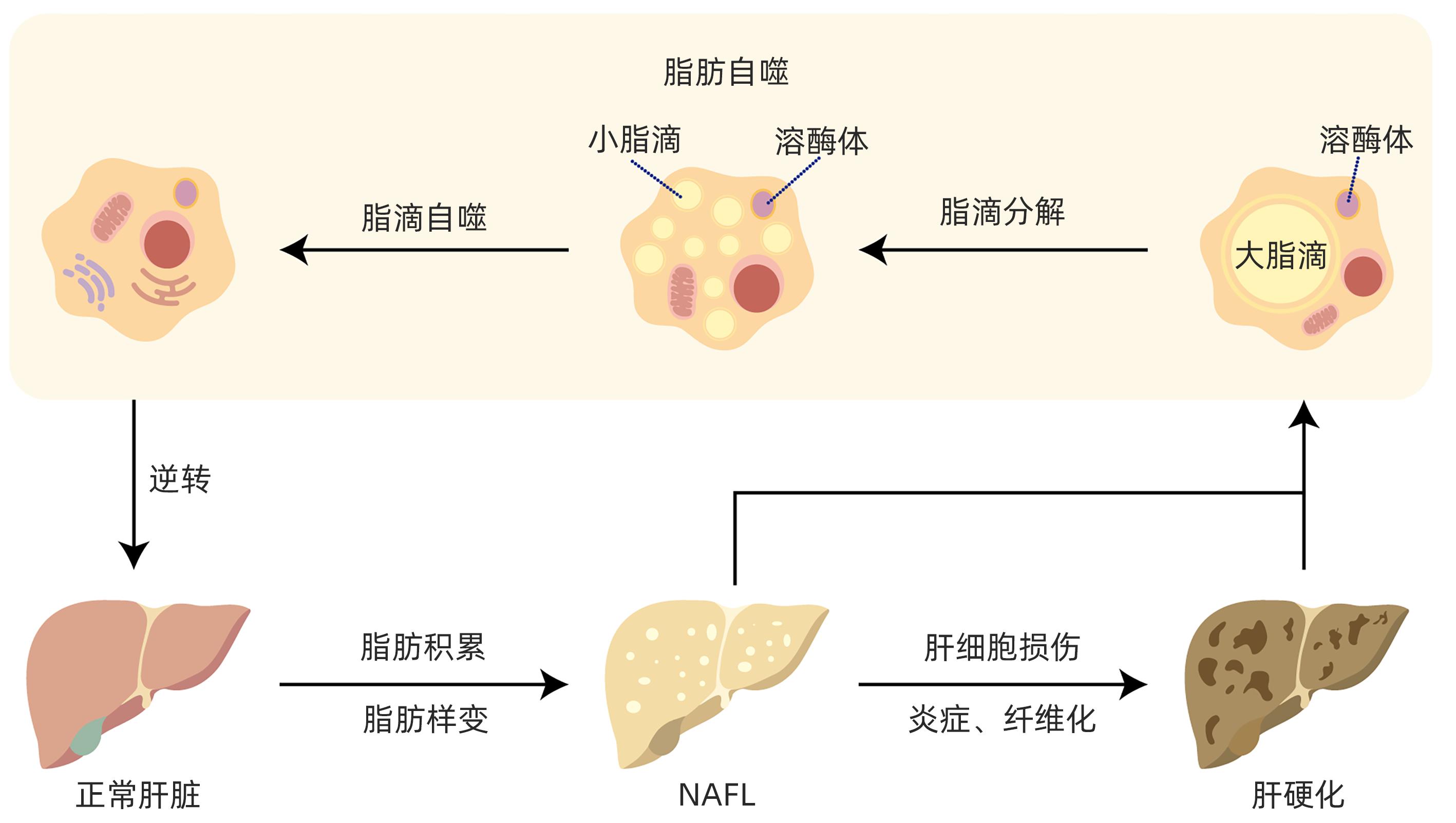
脂肪自噬在非酒精性脂肪性肝病防治中的作用
DOI: 10.12449/JCH240725
Role of lipophagy in the prevention and treatment of nonalcoholic fatty liver disease
-
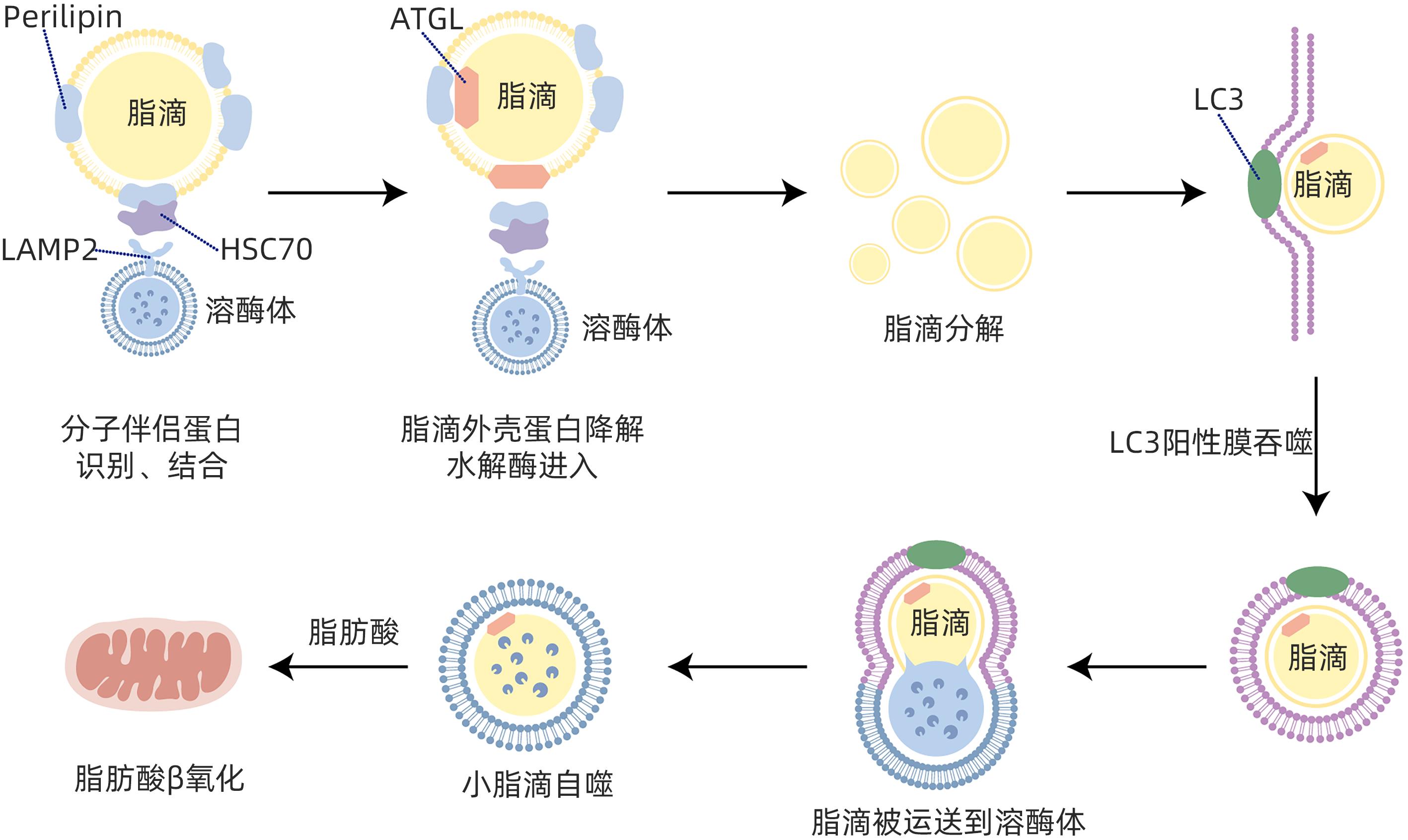
摘要: 目前非酒精性脂肪性肝病在国内外流行趋势不断上升,发病率逐年增加,已严重影响人类生命健康。脂肪自噬是分子伴侣介导的自噬,具有促进脂肪分解、维持肝细胞脂质稳态、缓解肝细胞脂肪变性等作用。脂肪自噬主要包括脂滴分解、脂滴自噬和脂肪酸β氧化三个过程,受关键基因、受体、酶调控。目前中药、西药及饮食运动等干预手段在脂肪自噬研究方面取得了重要进展,为非酒精性脂肪性肝病防治策略提供了新的视角。Abstract: Nowadays, the prevalence of nonalcoholic fatty liver disease (NAFLD) is constantly rising in China and globally, and its incidence rate is increasing year by year, which has seriously affected human life and health. Lipophagy is molecular chaperone-mediated autophagy and has the functions of promoting lipolysis, maintaining the lipid homeostasis of hepatocytes, and alleviating hepatocyte fatty degeneration. Lipophagy has three main processes of lipid droplet catabolism, lipid droplet autophagy, and fatty acid β-oxidation, which are regulated by key genes, receptors, and enzymes. Currently, important advances have been achieved for the intervention methods of traditional Chinese medicine, Western medicine, diet, and exercise in the research on lipophagy, which provides new perspectives for the prevention and treatment strategies for NAFLD.
-
Key words:
- Non-alcoholic Fatty Liver Disease /
- Autophagy /
- Therapeutics
-
表 1 不同靶点对脂肪自噬的影响及在NAFLD中的可能作用机制
Table 1. Effect of different targets on lipophagy and possible mechanisms of action in NAFLD
靶点 类型 靶点 影响 相关作用机制 基因 TFEB[16] 增强 通过PGC-1α和PPARα调节参与脂质代谢相关基因 TFE3[17] 增强 激活PGC-lα介导的脂肪酸β氧化 IRGM[18] 增强 敲除IRGM抑制细胞中的自噬通量 SRSF3[19] 增强 SRSF3的缺失增加STX17的泛素化和降解,阻断自噬体和溶酶体的融合 LXRα[20] 减弱 转录激活microRNA let-7a-2和microRNA 34a基因,下调ATG4B和Rab-8B 抑制肝细胞中的自噬 C9ORF72[21] 减弱 激活Cdc42/N-WASP轴,抑制脂肪自噬 受体 ORP8[22] 增强 ORP8与LC3/GABARAP相互作用,充当递送脂滴的受体 SQSTM1/p62[23] 增强 (1)作为非排他性脂肪自噬受体;(2)影响溶酶体中脂滴丰度 CD36[24-25] 减弱 与AMPK途径及ULK1/Beclin1的磷酸化有关 P2RX7[26] 增强 激活AMPK/ULK1途径 mTORC1-Plin3[27] 增强 mTORC1和脂滴包衣蛋白Plin3可作为对接蛋白,促进Plin3磷酸化 酶 ATGL 增强 促进脂肪分解过程 STX11[28] 减弱 (1)调节ATGL的空间分布;(2)削弱ATGL在肝细胞中的作用 IFGGA2[29] 增强 (1)IFGGA2与ATGL相互作用;(2)增强LC3B与脂滴的结合 AMPK 增强 促进脂肪自噬过程 SIRT3[30] 增强 (1)激活AMPK和ULK1;(2)刺激LAMP2A-HSC70-PLN2复合物的形成 SCD1[31] 减弱 抑制肝细胞中SCD1表达可增强AMPK活性 过氧化物酶体[32] 减弱 促进RPTOR的乙酰化,激活mTOR活化抑制脂肪自噬 表 2 不同干预手段基于脂肪自噬作用防治NAFLD的研究
Table 2. Different interventions against NAFLD based on the role of lipophagy
干预 相关靶点或通路 主要结论 西药 地高辛与阿培利司[33] 尚未明确 通过脂肪自噬降低肝脂肪变性,减弱炎症和促纤维化基因的表达,抑制肝纤维化 二甲双胍[34] AMPK; AMPK-SIRT1轴 通过AMPK介导的氧化降低血浆游离脂肪酸,诱导自噬和自噬通量,AMPK-SIRT1轴诱导三四脯氨酸活化,以减少肝细胞坏死性凋亡;同时破坏肝细胞中脑内富含Ras同源物mRNA的稳定性来增加脂肪自噬 非诺贝特[35] CaMKKβ-AMPK-ULK1途径; TFEB;TFE3;mTOR 通过激活钙调磷酸酶和CaMKKβ-AMPK-ULK1途径,促进TFEB、TFE3去磷酸化和核易位,并以mTOR非依赖性方式减少肝脂肪堆积 生长分化因子11[36] 尚未明确 上调细胞自噬,消除高脂导致的肝细胞脂质积累,并减少ROS,提升线粒体膜电位,从而改善NAFLD 培贝夫明[37] FGF21 FGF21参与了脂肪生成、糖摄取、β氧化、炎症和纤维化等过程的调节,可显著减少NASH患者的肝脂肪 p62激动剂[38] p62 通过N-degron促进脂肪自噬,治疗小鼠肝脂肪病和肥胖症 CAY10566[31] SCD1 增强的AMPK活性促进脂肪吞噬,显著减少肝脂肪变性和肝脂滴积聚 中药有效活性成分 川陈皮素[39] TFEB 通过TFEB介导的溶酶体生物发生和脂肪自噬来缓解肝脂肪变性,减少NOD样受体蛋白3炎症小体组装,调节体内外M1/M2巨噬细胞极化,从而减轻NAFLD 益母草苷[40] TFEB 促进TFEB介导的脂肪自噬以缓解NAFLD 尚未明确 通过脂肪自噬途径诱导肝脂肪清除 青钱柳提取物[41] 连翘脂素[42] 钙调磷酸酶-TFEB轴 通过调节肝细胞中的钙调磷酸酶-TFEB轴来恢复脂肪自噬,并抑制脂质积累和炎症 柚皮苷[43] TFEB 促进自噬体和溶酶体的融合,恢复受损的自噬通量,并进一步诱导脂肪自噬,减轻肝脂肪变性 白藜芦醇苷[44] mTOR;TFEB 抑制mTOR信号传导并上调TFEB的表达和活性,恢复自噬通量改善NASH 芒柄花黄素[45] AMPK;TFEB 激活AMPK并促进TFEB的后续核易位,从而改善小鼠的肝脂肪变性 萝卜硫素[46-47] AMPK-mTOR-ULK1通路 Nrf2依赖性方式刺激脂肪自噬来增强脂滴降解,同时通过AMPK-mTOR-ULK1通路促进噬脂作用,改善肝细胞中脂滴的过度积累 人参皂苷化合物K[48] AMPK/ULK1途径;ATGL 与糖皮质激素受体结合,通过AMPK/ULK1途径激活脂肪自噬,同时促进糖皮质激素受体与ATGL启动子的结合,增加脂肪酶表达 和厚朴酚[49] SIRT3-AMPK-脂肪自噬轴 激活SIRT3-AMPK-脂肪自噬轴来改善肝细胞的脂毒性 槲皮素[50-51] AMPK 激活AMPK信号传导,促进脂肪自噬以产生线粒体中脂肪酸β氧化的底物;降低Perilipin 2水平,激活AMPK活性促进脂肪自噬,减轻肝脏脂肪的积累 甜叶菊和甜菊糖苷[52] PPARα 通过PPARα介导的脂肪自噬,减轻肝细胞中的肝脂肪变性 缬草及其环烯醚萜[53] mTORC1;Atg5 作为自噬增强剂来分解脂滴,通过抑制mTORC1活性诱导自噬,同时以Atg5依赖性方式减少脂质积累 饮食 高碳水化合物[55] ROS-AKT-Beclin1途径 肠道脂质积聚后,通过ROS-AKT-Beclin1途径激活肠上皮细胞中的脂肪自噬,减轻葡萄糖诱导的脂质积累 高磷饮食[56] AMPK 激活AMPK通路和上调Beclin1磷酸化水平,促进脂肪自噬来减少肝脂沉积 禁食[57] FGF21 诱导FGF21信号转导,通过组蛋白去甲基化酶激活肝脏脂肪自噬和脂质降解 饥饿后进食[58] 尚未明确 激活肠道中的脂肪自噬 运动 跑步运动[60] FITM2、CIDEA和FSP27基因 降低脂滴生成以及脂滴扩张相关基因FITM2、CIDEA和FSP27的表达,促进LAMP1与脂滴的共定位,抑制异常脂滴膨胀和增强溶酶体噬脂作用,从而调节脂滴的生物发生和自噬以缓解NAFLD 游泳运动[61] AMPK/SIRT1 刺激AMPK/SIRT1激活肝脏的脂肪自噬,从而降低肝脂肪变性和胰岛素抵抗 -
[1] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. [2] RIAZI K, AZHARI H, CHARETTE JH, et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 851- 861. DOI: 10.1016/S2468-1253(22)00165-0. [3] ZHOU F, ZHOU JH, WANG WX, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: A systematic review and meta-analysis[J]. Hepatology, 2019, 70( 4): 1119- 1133. DOI: 10.1002/hep.30702. [4] LAVAL T, OUIMET M. A role for lipophagy in atherosclerosis[J]. Nat Rev Cardiol, 2023, 20( 7): 431- 432. DOI: 10.1038/s41569-023-00885-z. [5] GLICK D, BARTH S, MACLEOD KF. Autophagy: Cellular and molecular mechanisms[J]. J Pathol, 2010, 221( 1): 3- 12. DOI: 10.1002/path.2697. [6] ZHOU KB, YAO PB, HE J, et al. Lipophagy in nonliver tissues and some related diseases: Pathogenic and therapeutic implications[J]. J Cell Physiol, 2019, 234( 6): 7938- 7947. DOI: 10.1002/jcp.27988. [7] SCHOTT MB, WELLER SG, SCHULZE RJ, et al. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes[J]. J Cell Biol, 2019, 218( 10): 3320- 3335. DOI: 10.1083/jcb.201803153. [8] ZHANG S, PENG XQ, YANG S, et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders[J]. Cell Death Dis, 2022, 13( 2): 132. DOI: 10.1038/s41419-022-04593-3. [9] KIM J, KUNDU M, VIOLLET B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nat Cell Biol, 2011, 13( 2): 132- 141. DOI: 10.1038/ncb2152. [10] EGAN DF, SHACKELFORD DB, MIHAYLOVA MM, et al. Phosphorylation of ULK1(hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy[J]. Science, 2011, 331( 6016): 456- 461. DOI: 10.1126/science.1196371. [11] ZHANG ZL, YAO Z, CHEN YF, et al. Lipophagy and liver disease: New perspectives to better understanding and therapy[J]. Biomed Pharmacother, 2018, 97: 339- 348. DOI: 10.1016/j.biopha.2017.07.168. [12] SCHULZE RJ, MCNIVEN MA. Lipid droplet formation and lipophagy in fatty liver disease[J]. Semin Liver Dis, 2019, 39( 3): 283- 290. DOI: 10.1055/s-0039-1685524. [13] JONAS W, SCHWERBEL K, ZELLNER L, et al. Alterations of lipid profile in livers with impaired lipophagy[J]. Int J Mol Sci, 2022, 23( 19): 11863. DOI: 10.3390/ijms231911863. [14] HAN SL, QIAN YC, LIMBU SM, et al. Lipolysis and lipophagy play individual and interactive roles in regulating triacylglycerol and cholesterol homeostasis and mitochondrial form in zebrafish[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2021, 1866( 9): 158988. DOI: 10.1016/j.bbalip.2021.158988. [15] ROBICHAUD S, FAIRMAN G, VIJITHAKUMAR V, et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells[J]. Autophagy, 2021, 17( 11): 3671- 3689. DOI: 10.1080/15548627.2021.1886839. [16] SETTEMBRE C, de CEGLI R, MANSUETO G, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop[J]. Nat Cell Biol, 2013, 15( 6): 647- 658. DOI: 10.1038/ncb2718. [17] XIONG J, WANG KZ, HE JP, et al. TFE3 alleviates hepatic steatosis through autophagy-induced lipophagy and PGC1α-mediated fatty acid β-oxidation[J]. Int J Mol Sci, 2016, 17( 3): 387. DOI: 10.3390/ijms17030387. [18] LIN YC, CHANG PF, LIN HF, et al. Variants in the autophagy-related gene IRGM confer susceptibility to non-alcoholic fatty liver disease by modulating lipophagy[J]. J Hepatol, 2016, 65( 6): 1209- 1216. DOI: 10.1016/j.jhep.2016.06.029. [19] LI Y, WANG T, LIAO QM, et al. Loss of splicing factor SRSF3 impairs lipophagy through ubiquitination and degradation of Syntaxin17 in hepatocytes[J]. J Lipid Res, 2023, 64( 3): 100342. DOI: 10.1016/j.jlr.2023.100342. [20] KIM YS, NAM HJ, HAN CY, et al. Liver X receptor alpha activation inhibits autophagy and lipophagy in hepatocytes by dysregulating autophagy-related 4B cysteine peptidase and Rab-8B, reducing mitochondrial fuel oxidation[J]. Hepatology, 2021, 73( 4): 1307- 1326. DOI: 10.1002/hep.31423. [21] CANG XM, WANG Y, ZENG J, et al. C9orf72 knockdown alleviates hepatic insulin resistance by promoting lipophagy[J]. Biochem Biophys Res Commun, 2022, 588: 15- 22. DOI: 10.1016/j.bbrc.2021.12.018. [22] WANG Z, ZHANG H. Join the club: ORP8 is a lipophagy receptor[J]. Protein Cell, 2023, 14( 9): 632- 634. DOI: 10.1093/procel/pwad005. [23] SHROFF A, NAZARKO TY. SQSTM1, lipid droplets and current state of their lipophagy affairs[J]. Autophagy, 2023, 19( 2): 720- 723. DOI: 10.1080/15548627.2022.2094606. [24] LI Y, YANG P, ZHAO L, et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway[J]. J Lipid Res, 2019, 60( 4): 844- 855. DOI: 10.1194/jlr.M090969. [25] ZHAO L, ZHANG C, LUO XX, et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis[J]. J Hepatol, 2018, 69( 3): 705- 717. DOI: 10.1016/j.jhep.2018.04.006. [26] DONG ZZ, WEI YJ, TAO M, et al. Activation of the purinergic receptor P2X7 improves hepatosteatosis by promoting lipophagy[J]. FEBS Lett, 2021, 595( 22): 2768- 2780. DOI: 10.1002/1873-3468.14207. [27] GARCIA-MACIA M, SANTOS-LEDO A, LESLIE J, et al. A mammalian target of rapamycin-perilipin 3(mTORC1-Plin3) pathway is essential to activate lipophagy and protects against hepatosteatosis[J]. Hepatology, 2021, 74( 6): 3441- 3459. DOI: 10.1002/hep.32048. [28] ZHANG GJ, HAN JX, WANG LL, et al. The vesicular transporter STX11 governs ATGL-mediated hepatic lipolysis and lipophagy[J]. iScience, 2022, 25( 4): 104085. DOI: 10.1016/j.isci.2022.104085. [29] SCHWERBEL K, KAMITZ A, KRAHMER N, et al. Immunity-related GTPase induces lipophagy to prevent excess hepatic lipid accumulation[J]. J Hepatol, 2020, 73( 4): 771- 782. DOI: 10.1016/j.jhep.2020.04.031. [30] ZHANG T, LIU JX, SHEN SN, et al. SIRT3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity[J]. Cell Death Differ, 2020, 27( 1): 329- 344. DOI: 10.1038/s41418-019-0356-z. [31] ZHOU YP, ZHONG L, YU SJ, et al. Inhibition of stearoyl-coenzyme A desaturase 1 ameliorates hepatic steatosis by inducing AMPK-mediated lipophagy[J]. Aging(Albany NY), 2020, 12( 8): 7350- 7362. DOI: 10.18632/aging.103082. [32] HE AY, DEAN JM, LU DL, et al. Hepatic peroxisomal β-oxidation suppresses lipophagy via RPTOR acetylation and MTOR activation[J]. Autophagy, 2020, 16( 9): 1727- 1728. DOI: 10.1080/15548627.2020.1797288. [33] MINAMI Y, HOSHINO A, HIGUCHI Y, et al. Liver lipophagy ameliorates nonalcoholic steatohepatitis through extracellular lipid secretion[J]. Nat Commun, 2023, 14( 1): 4084. DOI: 10.1038/s41467-023-39404-6. [34] PARK J, RAH SY, AN HS, et al. Metformin-induced TTP mediates communication between Kupffer cells and hepatocytes to alleviate hepatic steatosis by regulating lipophagy and necroptosis[J]. Metabolism, 2023, 141: 155516. DOI: 10.1016/j.metabol.2023.155516. [35] YOO J, JEONG IK, AHN KJ, et al. Fenofibrate, a PPARα agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy[J]. Metabolism, 2021, 120: 154798. DOI: 10.1016/j.metabol.2021.154798. [36] ZHANG T, YIN XC, REN FF, et al. Mechanism of growth differentiation factor 11 regulating nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 9): 2103- 2109. DOI: 10.3969/j.issn.1001-5256.2023.09.011.张涛, 殷雪翠, 任飞飞, 等. 生长分化因子11(GDF11)调节非酒精性脂肪性肝病的机制研究[J]. 临床肝胆病杂志, 2023, 39( 9): 2103- 2109. DOI: 10.3969/j.issn.1001-5256.2023.09.011. [37] TUCKER B, LI HT, LONG XX, et al. Fibroblast growth factor 21 in non-alcoholic fatty liver disease[J]. Metabolism, 2019, 101: 153994. DOI: 10.1016/j.metabol.2019.153994. [38] JUNG EJ, SUNG KW, BAE TH, et al. The N-degron pathway mediates lipophagy: The chemical modulation of lipophagy in obesity and NAFLD[J]. Metabolism, 2023, 146: 155644. DOI: 10.1016/j.metabol.2023.155644. [39] YANG XS, DENG YD, TU YL, et al. Nobiletin mitigates NAFLD via lipophagy and inflammation[J]. Food Funct, 2022, 13( 19): 10186- 10199. DOI: 10.1039/d2fo01682f. [40] ZHANG H, LU JF, LIU H, et al. Ajugol enhances TFEB-mediated lysosome biogenesis and lipophagy to alleviate non-alcoholic fatty liver disease[J]. Pharmacol Res, 2021, 174: 105964. DOI: 10.1016/j.phrs.2021.105964. [41] YANG WW, JIANG CH, WANG ZG, et al. Cyclocarya paliurus extract attenuates hepatic lipid deposition in HepG2 cells by the lipophagy pathway[J]. Pharm Biol, 2020, 58( 1): 838- 844. DOI: 10.1080/13880209.2020.1803365. [42] ZHOU WL, YAN X, ZHAI YY, et al. Phillygenin ameliorates nonalcoholic fatty liver disease via TFEB-mediated lysosome biogenesis and lipophagy[J]. Phytomedicine, 2022, 103: 154235. DOI: 10.1016/j.phymed.2022.154235. [43] GUAN LL, GUO L, ZHANG H, et al. Naringin protects against non-alcoholic fatty liver disease by promoting autophagic flux and lipophagy[J]. Mol Nutr Food Res, 2024, 68( 3): e2200812. DOI: 10.1002/mnfr.202200812. [44] CHEN XT, CHAN H, ZHANG L, et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux[J]. J Cell Mol Med, 2019, 23( 6): 4290- 4300. DOI: 10.1111/jcmm.14320. [45] WANG Y, ZHAO HL, LI X, et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy[J]. J Nutr Biochem, 2019, 73: 108214. DOI: 10.1016/j.jnutbio.2019.07.005. [46] LEI P, HU YQ, GAO P, et al. Sulforaphane ameliorates hepatic lipid metabolism via modulating lipophagy in vivo and in vitro[J]. J Agric Food Chem, 2022, 70( 48): 15126- 15133. DOI: 10.1021/acs.jafc.2c06311. [47] MASUDA M, YOSHIDA-SHIMIZU R, MORI Y, et al. Sulforaphane induces lipophagy through the activation of AMPK-mTOR-ULK1 pathway signaling in adipocytes[J]. J Nutr Biochem, 2022, 106: 109017. DOI: 10.1016/j.jnutbio.2022.109017. [48] YANG SW, LIU T, HU CX, et al. Ginsenoside compound K protects against obesity through pharmacological targeting of glucocorticoid receptor to activate lipophagy and lipid metabolism[J]. Pharmaceutics, 2022, 14( 6): 1192. DOI: 10.3390/pharmaceutics14061192. [49] LIU JX, ZHANG T, ZHU JZ, et al. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy[J]. Chin Med, 2021, 16( 1): 115. DOI: 10.1186/s13020-021-00528-w. [50] FUKAYA M, SATO Y, KONDO S, et al. Quercetin enhances fatty acid β-oxidation by inducing lipophagy in AML12 hepatocytes[J]. Heliyon, 2021, 7( 6): e07324. DOI: 10.1016/j.heliyon.2021.e07324. [51] ZENG HM, GUO XP, ZHOU F, et al. Quercetin alleviates ethanol-induced liver steatosis associated with improvement of lipophagy[J]. Food Chem Toxicol, 2019, 125: 21- 28. DOI: 10.1016/j.fct.2018.12.028. [52] PARK M, SHARMA A, BAEK H, et al. Stevia and stevioside attenuate liver steatosis through PPARα-mediated lipophagy in db/db mice hepatocytes[J]. Antioxidants(Basel), 2022, 11( 12): 2496. DOI: 10.3390/antiox11122496. [53] LEE DH, PARK SH, HUH YH, et al. Iridoids of Valeriana fauriei contribute to alleviating hepatic steatosis in obese mice by lipophagy[J]. Biomed Pharmacother, 2020, 125: 109950. DOI: 10.1016/j.biopha.2020.109950. [54] GAO Y, ZHANG W, ZENG LQ, et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy[J]. Redox Biol, 2020, 36: 101635. DOI: 10.1016/j.redox.2020.101635. [55] WU LX, XU YC, HOGSTRAND C, et al. Lipophagy mediated glucose-induced changes of lipid deposition and metabolism via ROS dependent AKT-Beclin1 activation[J]. J Nutr Biochem, 2022, 100: 108882. DOI: 10.1016/j.jnutbio.2021.108882. [56] LIU XY, ZHAO T, WEI XL, et al. Dietary phosphorus reduced hepatic lipid deposition by activating ampk pathway and Beclin1 phosphorylation levels to activate lipophagy in Tilapia Oreochromis niloticus[J]. Front Nutr, 2022, 9: 841187. DOI: 10.3389/fnut.2022.841187. [57] BYUN S, SEOK S, KIM YC, et al. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase[J]. Nat Commun, 2020, 11( 1): 807. DOI: 10.1038/s41467-020-14384-z. [58] RAIMUNDO N. Feeding and lipophagy: It takes guts to deliver[J]. EMBO J, 2022, 41( 17): e112180. DOI: 10.15252/embj.2022112180. [59] LI RD, LI GK, HAI Y, et al. The effect of aerobic exercise on the lipophagy of adipose tissue in obese male mice[J]. Chem Phys Lipids, 2022, 247: 105225. DOI: 10.1016/j.chemphyslip.2022.105225. [60] YANG YJ, LI X, LIU ZH, et al. Moderate treadmill exercise alleviates NAFLD by regulating the biogenesis and autophagy of lipid droplet[J]. Nutrients, 2022, 14( 22): 4910. DOI: 10.3390/nu14224910. [61] LI H, DUN YS, ZHANG WL, et al. Exercise improves lipid droplet metabolism disorder through activation of AMPK-mediated lipophagy in NAFLD[J]. Life Sci, 2021, 273: 119314. DOI: 10.1016/j.lfs.2021.119314. -



 PDF下载 ( 1004 KB)
PDF下载 ( 1004 KB)

 下载:
下载: