固醇调节元件结合蛋白(SREBP)在非酒精性脂肪性肝病中的作用机制及治疗靶点
DOI: 10.12449/JCH240726
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:李安琪负责课题设计,资料分析,撰写论文;王锐负责修改论文;赵玉强、赵佩然参与收集及分析文献资料;杨婧负责拟定写作思路,指导撰写文章并最后定稿。
Mechanism of action of sterol regulatory element-binding proteins in nonalcoholic fatty liver disease and related therapeutic targets
-
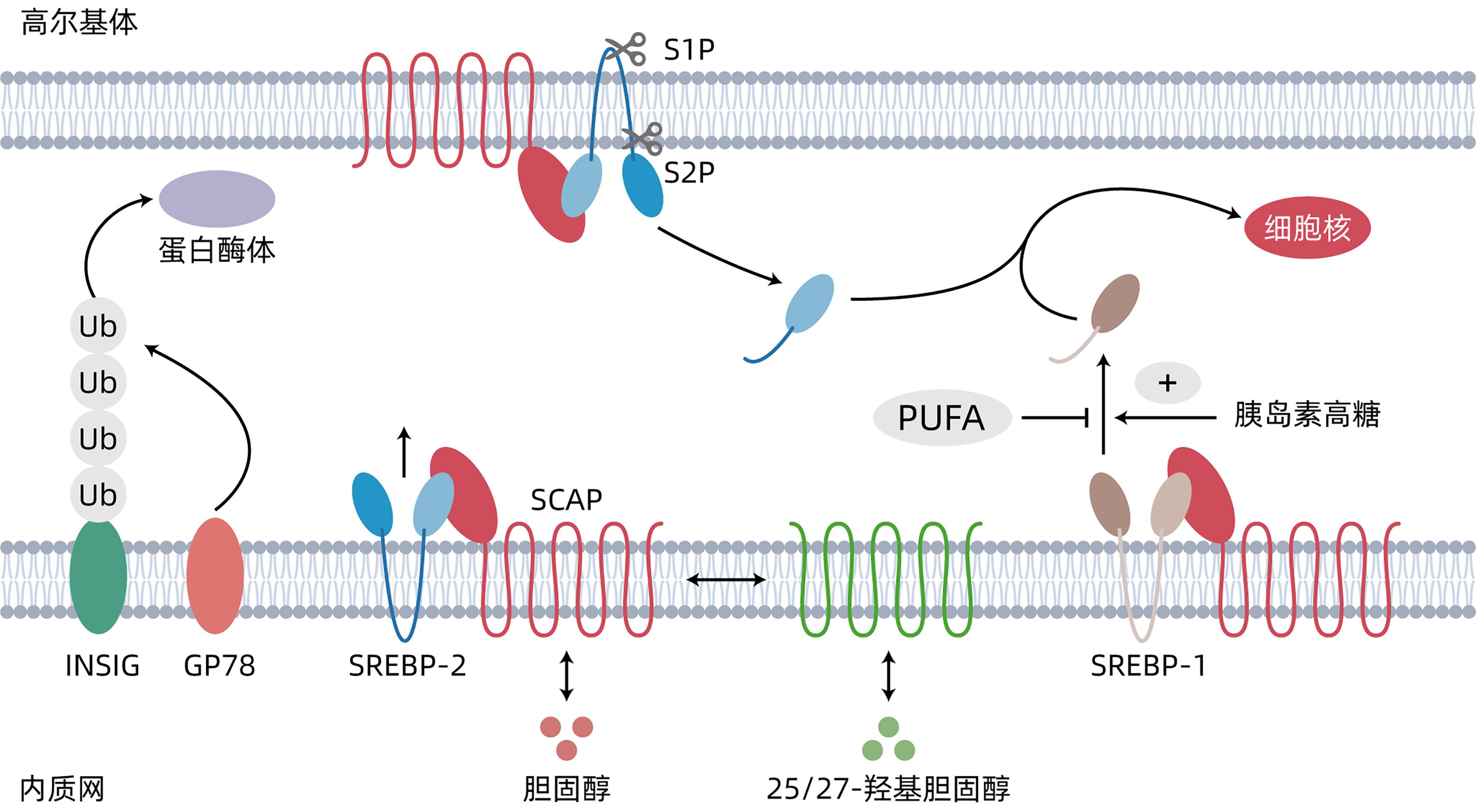
摘要: 非酒精性脂肪性肝病(NAFLD)已成为全球范围内最常见的肝脏疾病,是肝癌发展的重要风险因素。然而,NAFLD的发病机制目前仍未被完全阐明,且尚无特异性的有效治疗措施。固醇调节元件结合蛋白(SREBP)是一类重要的核转录因子,主要通过激活胆固醇、脂肪酸和甘油三酯合成及摄取的相关基因来维持体内脂质代谢的平衡,是治疗代谢性疾病的靶点。本文对SREBP参与NAFLD发病机制的最新进展以及SREBP靶向治疗NAFLD的最新证据进行综述。值得注意的是,最近研究发现SREBP抑制与自噬受损共同引发肝损伤。因此,过度抑制脂肪生成对NAFLD的治疗可能起反作用。总体而言,SREBP是一个具有广阔前景的NAFLD治疗靶点,其对脂质代谢的分子机制受多种因素的调控,而这些因素正在被深入探索和分析,对NAFLD治疗具有重要的临床意义。
-
关键词:
- 非酒精性脂肪性肝病 /
- 胆固醇调节元件结合蛋白质类 /
- 脂肪生成 /
- 炎症 /
- 纤维化
Abstract: Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease in the world and is an important risk factor for the progression to hepatocellular carcinoma. However, the pathogenesis of NAFLD remains unclear, and there is still a lack of specific treatment measures. Sterol regulatory element-binding proteins (SREBP) are an important nuclear transcription factor, which mainly maintains the balance of lipid metabolism inside the body by activating the genes associated with the synthesis and uptake of cholesterol, fatty acids, and triglycerides, and therefore, SREBP are a target for the treatment of metabolic diseases. This article reviews the latest advances in SREBP in the pathogenesis of NAFLD and the latest evidence of SREBP-targeted therapy for NAFLD. It is worth noting that recent studies have shown that SREBP inhibition can cause liver injury together with autophagy damage. Therefore, excessive inhibition of lipogenesis may exert a counterproductive effect on the treatment of NAFLD. In conclusion, SREBP is a promising therapeutic target for NAFLD; the molecular mechanism of SREBP in lipid metabolism is regulated by many factors, and these factors are being deeply explored and analyzed, which has an important clinical significance for the treatment of NAFLD. -
[1] MUNDI MS, VELAPATI S, PATEL J, et al. Evolution of NAFLD and its management[J]. Nutr Clin Pract, 2020, 35( 1): 72- 84. DOI: 10.1002/ncp.10449. [2] KAUFMANN B, RECA A, WANG B, et al. Mechanisms of nonalcoholic fatty liver disease and implications for surgery[J]. Langenbecks Arch Surg, 2021, 406( 1): 1- 17. DOI: 10.1007/s00423-020-01965-1. [3] POUWELS S, SAKRAN N, GRAHAM Y, et al. Non-alcoholic fatty liver disease(NAFLD): A review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22( 1): 63. DOI: 10.1186/s12902-022-00980-1. [4] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. [5] XU XH, POULSEN KL, WU LJ, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis(NAFL/NASH)[J]. Signal Transduct Target Ther, 2022, 7( 1): 287. DOI: 10.1038/s41392-022-01119-3. [6] GOLDSTEIN JL, DEBOSE-BOYD RA, BROWN MS. Protein sensors for membrane sterols[J]. Cell, 2006, 124( 1): 35- 46. DOI: 10.1016/j.cell.2005.12.022. [7] ARAKI M, NAKAGAWA Y, SAITO H, et al. Hepatocyte- or macrophage-specific SREBP-1a deficiency in mice exacerbates methionine- and choline-deficient diet-induced nonalcoholic fatty liver disease[J]. Am J Physiol Gastrointest Liver Physiol, 2022, 323( 6): G627- G639. DOI: 10.1152/ajpgi.00090.2022. [8] SATO R. Sterol metabolism and SREBP activation[J]. Arch Biochem Biophys, 2010, 501( 2): 177- 181. DOI: 10.1016/j.abb.2010.06.004. [9] NAKAGAWA Y, ARAKI M, HAN SI, et al. CREBH systemically regulates lipid metabolism by modulating and integrating cellular functions[J]. Nutrients, 2021, 13( 9): 3204. DOI: 10.3390/nu13093204. [10] JEON YG, KIM YY, LEE G, et al. Physiological and pathological roles of lipogenesis[J]. Nat Metab, 2023, 5( 5): 735- 759. DOI: 10.1038/s42255-023-00786-y. [11] MARGERIE D, LEFEBVRE P, RAVERDY V, et al. Hepatic transcriptomic signatures of statin treatment are associated with impaired glucose homeostasis in severely obese patients[J]. BMC Med Genomics, 2019, 12( 1): 80. DOI: 10.1186/s12920-019-0536-1. [12] IPSEN DH, LYKKESFELDT J, TVEDEN-NYBORG P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease[J]. Cell Mol Life Sci, 2018, 75( 18): 3313- 3327. DOI: 10.1007/s00018-018-2860-6. [13] GOLDSTEIN JL, BASU SK, BROWN MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells[J]. Methods Enzymol, 1983, 98: 241- 260. DOI: 10.1016/0076-6879(83)98152-1. [14] NOHTURFFT A, DEBOSE-BOYD RA, SCHEEK S, et al. Sterols regulate cycling of SREBP cleavage-activating protein(SCAP) between endoplasmic reticulum and Golgi[J]. Proc Natl Acad Sci U S A, 1999, 96( 20): 11235- 11240. DOI: 10.1073/pnas.96.20.11235. [15] HORTON JD, COHEN JC, HOBBS HH. Molecular biology of PCSK9: Its role in LDL metabolism[J]. Trends Biochem Sci, 2007, 32( 2): 71- 77. DOI: 10.1016/j.tibs.2006.12.008. [16] MUSSO G, GAMBINO R, CASSADER M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis[J]. Prog Lipid Res, 2013, 52( 1): 175- 191. DOI: 10.1016/j.plipres.2012.11.002. [17] CAROTTI S, AQUILANO K, ZALFA F, et al. Lipophagy impairment is associated with disease progression in NAFLD[J]. Front Physiol, 2020, 11: 850. DOI: 10.3389/fphys.2020.00850. [18] ALLAIRE M, RAUTOU PE, CODOGNO P, et al. Autophagy in liver diseases: Time for translation?[J]. J Hepatol, 2019, 70( 5): 985- 998. DOI: 10.1016/j.jhep.2019.01.026. [19] NGUYEN TTP, KIM DY, LEE YG, et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice[J]. Mol Cell, 2021, 81( 18): 3820- 3832. DOI: 10.1016/j.molcel.2021.06.003. [20] NGUYEN TTP, KIM DY, IM SS, et al. Impairment of ULK1 sulfhydration-mediated lipophagy by SREBF1/SREBP-1c in hepatic steatosis[J]. Autophagy, 2021, 17( 12): 4489- 4490. DOI: 10.1080/15548627.2021.1968608. [21] IM SS, OSBORNE TF. Protection from bacterial-toxin-induced apoptosis in macrophages requires the lipogenic transcription factor sterol regulatory element binding protein 1a[J]. Mol Cell Biol, 2012, 32( 12): 2196- 2202. DOI: 10.1128/MCB.06294-11. [22] LU D, PARISI LR, GOKCUMEN O, et al. SREBP activation contributes to fatty acid accumulations in necroptosis[J]. RSC Chem Biol, 2023, 4( 4): 310- 322. DOI: 10.1039/d2cb00172a. [23] GUO CS, CHI ZX, JIANG DL, et al. Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 inflammasome activation and cholesterol biosynthetic signaling in macrophages[J]. Immunity, 2018, 49( 5): 842- 856. DOI: 10.1016/j.immuni.2018.08.021. [24] PETTINELLI P, DEL POZO T, ARAYA J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: Correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion[J]. Biochim Biophys Acta, 2009, 1792( 11): 1080- 1086. DOI: 10.1016/j.bbadis.2009.08.015. [25] FUJII N, NARITA T, OKITA N, et al. Sterol regulatory element-binding protein-1c orchestrates metabolic remodeling of white adipose tissue by caloric restriction[J]. Aging Cell, 2017, 16( 3): 508- 517. DOI: 10.1111/acel.12576. [26] SOUNDARARAJAN R, WISHART AD, VASANTHA RUPASINGHE HP, et al. Quercetin 3-glucoside protects neuroblastoma(SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis[J]. J Biol Chem, 2008, 283( 4): 2231- 2245. DOI: 10.1074/jbc.M703583200. [27] SHIMANO H, SATO R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology[J]. Nat Rev Endocrinol, 2017, 13( 12): 710- 730. DOI: 10.1038/nrendo.2017.91. [28] DÜVEL K, YECIES JL, MENON S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1[J]. Mol Cell, 2010, 39( 2): 171- 183. DOI: 10.1016/j.molcel.2010.06.022. [29] KOHJIMA M, HIGUCHI N, KATO M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease[J]. Int J Mol Med, 2008, 21( 4): 507- 511. [30] PUIGSERVER P, RHEE J, DONOVAN J, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction[J]. Nature, 2003, 423( 6939): 550- 555. DOI: 10.1038/nature01667. [31] JUMP DB. Dietary polyunsaturated fatty acids and regulation of gene transcription[J]. Curr Opin Lipidol, 2002, 13( 2): 155- 164. DOI: 10.1097/00041433-200204000-00007. [32] GNONI A, GIUDETTI AM. Dietary long-chain unsaturated fatty acids acutely and differently reduce the activities of lipogenic enzymes and of citrate carrier in rat liver[J]. J Physiol Biochem, 2016, 72( 3): 485- 494. DOI: 10.1007/s13105-016-0495-3. [33] DONG QM, MAJUMDAR G, O’MEALLY RN, et al. Insulin-induced de novo lipid synthesis occurs mainly via mTOR-dependent regulation of proteostasis of SREBP-1c[J]. Mol Cell Biochem, 2020, 463( 1-2): 13- 31. DOI: 10.1007/s11010-019-03625-5. [34] FLISTER KFT, PINTO BAS, FRANÇA LM, et al. Long-term exposure to high-sucrose diet down-regulates hepatic endoplasmic reticulum-stress adaptive pathways and potentiates de novo lipogenesis in weaned male mice[J]. J Nutr Biochem, 2018, 62: 155- 166. DOI: 10.1016/j.jnutbio.2018.09.007. [35] YE J, RAWSON RB, KOMURO R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs[J]. Mol Cell, 2000, 6( 6): 1355- 1364. DOI: 10.1016/s1097-2765(00)00133-7. [36] LEE JS, ZHENG Z, MENDEZ R, et al. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model[J]. Toxicol Lett, 2012, 211( 1): 29- 38. DOI: 10.1016/j.toxlet.2012.02.017. [37] RÖHRL C, EIGNER K, WINTER K, et al. Endoplasmic reticulum stress impairs cholesterol efflux and synthesis in hepatic cells[J]. J Lipid Res, 2014, 55( 1): 94- 103. DOI: 10.1194/jlr.M043299. [38] GILARDI F, MIGLIAVACCA E, NALDI A, et al. Genome-wide analysis of SREBP1 activity around the clock reveals its combined dependency on nutrient and circadian signals[J]. PLoS Genet, 2014, 10( 3): e1004155. DOI: 10.1371/journal.pgen.1004155. [39] MARTELOT GL, CLAUDEL T, GATFIELD D, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis[J]. PLoS Biol, 2009, 7( 9): e1000181. DOI: 10.1371/journal.pbio.1000181. [40] GUAN DY, BAE H, ZHOU DS, et al. Hepatocyte SREBP signaling mediates clock communication within the liver[J]. J Clin Invest, 2023, 133( 8): e163018. DOI: 10.1172/JCI163018. [41] CHEN M, LIN YK, DANG YK, et al. Reprogramming of rhythmic liver metabolism by intestinal clock[J]. J Hepatol, 2023, 79( 3): 741- 757. DOI: 10.1016/j.jhep.2023.04.040. [42] SHAO W, MACHAMER CE, ESPENSHADE PJ. Fatostatin blocks ER exit of SCAP but inhibits cell growth in a SCAP-independent manner[J]. J Lipid Res, 2016, 57( 8): 1564- 1573. DOI: 10.1194/jlr.M069583. [43] LI YH, LUAN YS, LI JN, et al. Exosomal miR-199a-5p promotes hepatic lipid accumulation by modulating MST1 expression and fatty acid metabolism[J]. Hepatol Int, 2020, 14( 6): 1057- 1074. DOI: 10.1007/s12072-020-10096-0. [44] LI YH, NIE JJ, YANG YH, et al. Redox-unlockable nanoparticle-based MST1 delivery system to attenuate hepatic steatosis via the AMPK/SREBP-1c signaling axis[J]. ACS Appl Mater Interfaces, 2022, 14( 30): 34328- 34341. DOI: 10.1021/acsami.2c05889. [45] ASANO L, WATANABE M, RYODEN Y, et al. Vitamin D metabolite, 25-hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP[J]. Cell Chem Biol, 2017, 24( 2): 207- 217. DOI: 10.1016/j.chembiol.2016.12.017. [46] KAWAGOE F, MENDOZA A, HAYATA Y, et al. Discovery of a vitamin D receptor-silent vitamin D derivative that impairs sterol regulatory element-binding protein in vivo[J]. J Med Chem, 2021, 64( 9): 5689- 5709. DOI: 10.1021/acs.jmedchem.0c02179. [47] ISHIBASHI H, NAKAGAWA K, ONIMARU M, et al. Sp1 decoy transfected to carcinoma cells suppresses the expression of vascular endothelial growth factor, transforming growth factor beta1, and tissue factor and also cell growth and invasion activities[J]. Cancer Res, 2000, 60( 22): 6531- 6536. [48] AN HJ, KIM JY, GWON MG, et al. Beneficial effects of SREBP decoy oligodeoxynucleotide in an animal model of hyperlipidemia[J]. Int J Mol Sci, 2020, 21( 2): 552. DOI: 10.3390/ijms21020552. [49] JIANG SY, YANG XL, YANG ZM, et al. Discovery of an insulin-induced gene binding compound that ameliorates nonalcoholic steatohepatitis by inhibiting sterol regulatory element-binding protein-mediated lipogenesis[J]. Hepatology, 2022, 76( 5): 1466- 1481. DOI: 10.1002/hep.32381. [50] WANG YD, ZHANG JN, XU Z, et al. Identification and action mechanism of lipid regulating components from Rhei Radix et rhizoma[J]. J Ethnopharmacol, 2022, 292: 115179. DOI: 10.1016/j.jep.2022.115179. [51] SU ZL, HANG PZ, HU J, et al. Aloe-emodin exerts cholesterol-lowering effects by inhibiting proprotein convertase subtilisin/kexin type 9 in hyperlipidemic rats[J]. Acta Pharmacol Sin, 2020, 41( 8): 1085- 1092. DOI: 10.1038/s41401-020-0392-8. [52] MIYATA S, KODAKA M, KIKUCHI A, et al. Sulforaphane suppresses the activity of sterol regulatory element-binding proteins(SREBPs) by promoting SREBP precursor degradation[J]. Sci Rep, 2022, 12( 1): 8715. DOI: 10.1038/s41598-022-12347-6. [53] CHO WK, LEE MM, MA JY. Antiviral effect of isoquercitrin against influenza A viral infection via modulating hemagglutinin and neuraminidase[J]. Int J Mol Sci, 2022, 23( 21): 13112. DOI: 10.3390/ijms232113112. [54] KIM SH, YUN C, KWON D, et al. Effect of isoquercitrin on free fatty acid-induced lipid accumulation in HepG2 cells[J]. Molecules, 2023, 28( 3): 1476. DOI: 10.3390/molecules28031476. [55] NAJAFI-SHOUSHTARI SH, KRISTO F, LI YX, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis[J]. Science, 2010, 328( 5985): 1566- 1569. DOI: 10.1126/science.1189123. [56] HORIE T, NISHINO T, BABA O, et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice[J]. Nat Commun, 2013, 4: 2883. DOI: 10.1038/ncomms3883. [57] LI LF, ZHANG XY, REN HJ, et al. MiR-23a/b-3p promotes hepatic lipid accumulation by regulating Srebp-1c and Fas[J]. J Mol Endocrinol, 2021, 68( 1): 35- 49. DOI: 10.1530/JME-20-0324. [58] SOBKY SA EL, ABOUD NK, ASSALY NM EL, et al. Regulation of lipid droplet(LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs[J]. Front Med, 2022, 9: 903856. DOI: 10.3389/fmed.2022.903856. [59] CHENG CW, DENG XL, XU KS. Increased expression of sterol regulatory element binding protein-2 alleviates autophagic dysfunction in NAFLD[J]. Int J Mol Med, 2018, 41( 4): 1877- 1886. DOI: 10.3892/ijmm.2018.3389. [60] DENG XL, PAN XL, CHENG CW, et al. Regulation of SREBP-2 intracellular trafficking improves impaired autophagic flux and alleviates endoplasmic reticulum stress in NAFLD[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2017, 1862( 3): 337- 350. DOI: 10.1016/j.bbalip.2016.12.007. [61] JU UI, JEONG DW, SEO J, et al. Neddylation of sterol regulatory element-binding protein 1c is a potential therapeutic target for nonalcoholic fatty liver treatment[J]. Cell Death Dis, 2020, 11( 4): 283. DOI: 10.1038/s41419-020-2472-6. [62] SU SY, TIAN HM, JIA X, et al. Mechanistic insights into the effects of SREBP1c on hepatic stellate cell and liver fibrosis[J]. J Cell Mol Med, 2020, 24( 17): 10063- 10074. DOI: 10.1111/jcmm.15614. [63] KAWAMURA S, MATSUSHITA Y, KUROSAKI S, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis[J]. J Clin Invest, 2022, 132( 11): e151895. DOI: 10.1172/JCI151895. -



 PDF下载 ( 914 KB)
PDF下载 ( 914 KB)

 下载:
下载:


