非酒精性脂肪性肝病发生进展期肝纤维化的危险因素及列线图预测模型构建
DOI: 10.12449/JCH240812
Risk factors for the development of advanced liver fibrosis in nonalcoholic fatty liver disease and establishment of a nomogram model
-
摘要:
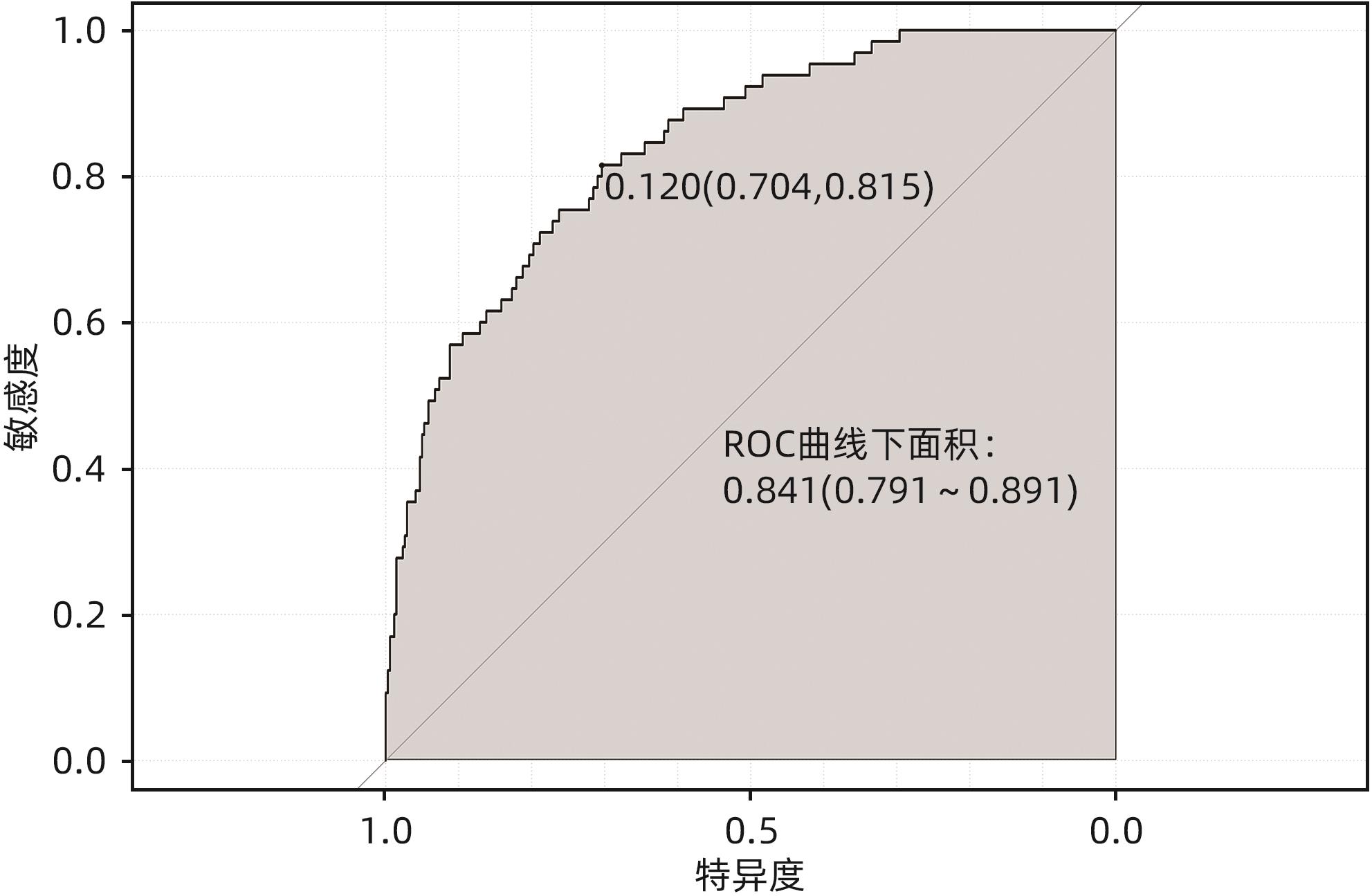
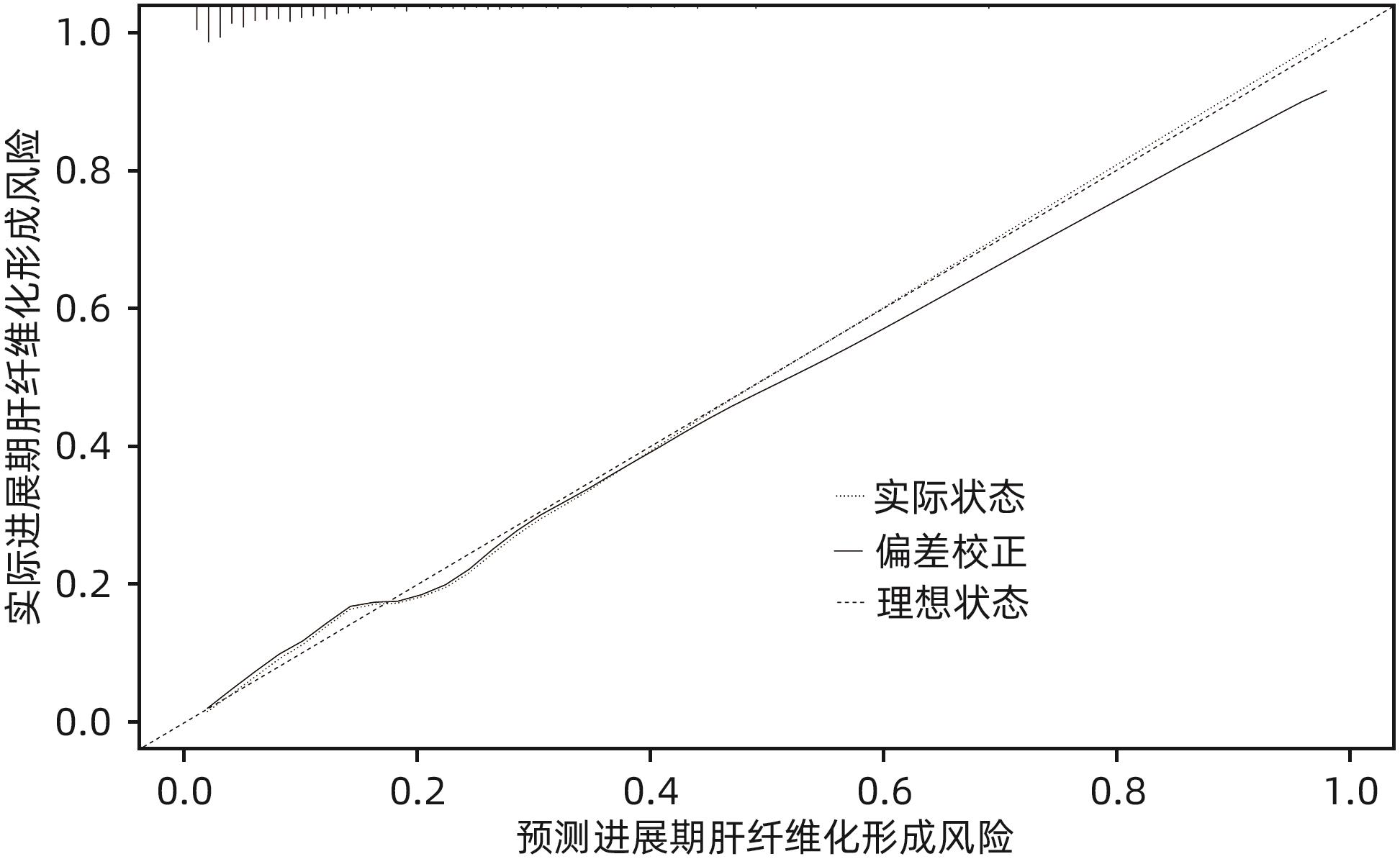
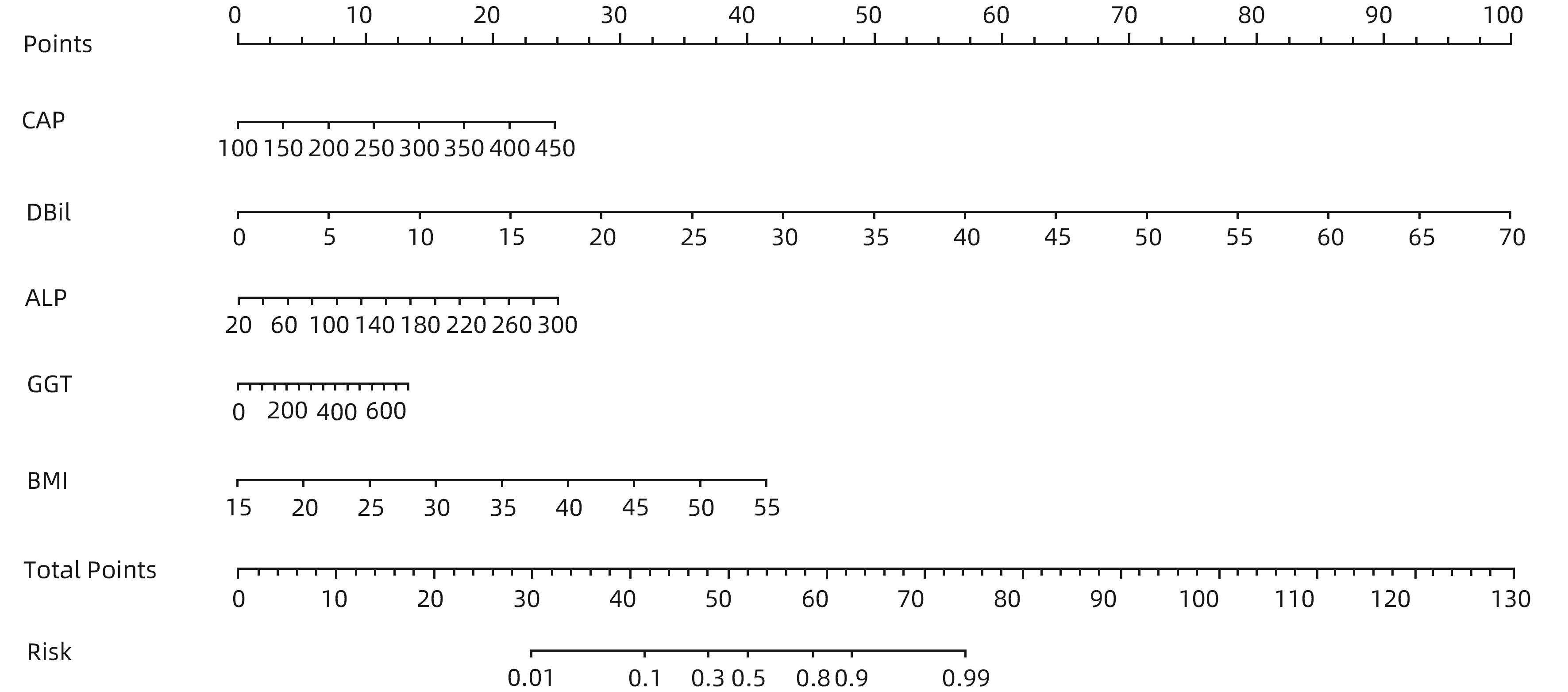
目的 通过分析非酒精性脂肪性肝病(NAFLD)进展期肝纤维化患者的临床特征,探讨进展期肝纤维化发生的危险因素,并建立预测进展期肝纤维化发生风险的列线图。 方法 回顾性分析2022年1月—2023年10月就诊于河南中医药大学第一附属医院的406例NAFLD患者的临床资料,依据FibroScan检测的肝脏弹性值(LSM)是否≥11.0 kPa,分为进展期肝纤维化组(65例)和非进展期纤维化组(341例),收集患者一般资料、实验室检查指标、既往病史。正态分布的计量资料两组间比较采用成组t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。通过多因素Logistic回归分析筛选独立危险因素,并基于此建立列线图,采用受试者工作特征曲线(ROC曲线)评估该列线图模型的区分度,校准曲线评价其有效性。 结果 单因素分析显示,进展期肝纤维化组患者的年龄、受控衰减参数、总胆红素、直接胆红素、间接胆红素、球蛋白、丙氨酸氨基转移酶、门冬氨酸氨基转移酶、碱性磷酸酶、谷氨酰转移酶、葡萄糖、体质量指数、糖尿病史与非进展期肝纤维化组比较,差异均有统计学意义(P值均<0.05)。进一步通过Logistic回归分析发现受控衰减参数(OR=1.015,95%CI:1.006~1.024,P=0.010)、直接胆红素(OR=1.345,95%CI:1.139~1.590,P=0.001)、碱性磷酸酶(OR=1.019,95%CI:1.008~1.029,P=0.001)、谷氨酰转移酶(OR=1.004,95%CI:1.000~1.008,P=0.035)和身体质量指数(OR=1.240,95%CI:1.137~1.353,P=0.001)是NAFLD进展期肝纤维化发生的独立危险因素。基于多因素回归结果构建列线图,ROC曲线分析结果显示该列线图模型预测NAFLD人群发生进展期肝纤维化的曲线下面积为0.841(95%CI:0.791~0.891);校准曲线显示进展期肝纤维化发生的观测值与预测值拟合度较好。 结论 受控衰减参数、身体质量指数、直接胆红素、碱性磷酸酶及谷氨酰转移酶水平升高是NAFLD进展期肝纤维化的独立影响因素,基于此建立的列线图预测效能良好,对进展期肝纤维化具有一定的预测价值。 Abstract:Objective To investigate the risk factors for the development of advanced liver fibrosis by analyzing the clinical features of patients with in nonalcoholic fatty liver disease (NAFLD) and advanced liver fibrosis, and to establish a nomogram model for predicting the risk of advanced liver fibrosis. Methods A retrospective analysis was performed for the clinical data of 406 NAFLD patients who attended The First Affiliated Hospital of Henan University of Chinese Medicine from January 2022 to October 2023, and according to whether liver stiffness measurement (LSM) measured by FibroScan was ≥11.0 kPa, the patients were divided into advanced liver fibrosis group with 65 patients and non-advanced liver fibrosis group with 341 patients. Related data were collected, including general information, laboratory markers, and medical history. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. A multivariate Logistic regression analysis was used to identify independent risk factors, and a nomogram model was established based on these factors. The receiver operating characteristic (ROC) curve was used to evaluate the discriminatory ability of the nomogram model, and the calibration curve was used to evaluate its effectiveness. Results The univariate analysis showed that there were significant differences between the advanced liver fibrosis group and the non-advanced liver fibrosis group in age, controlled attenuation parameter (CAP), total bilirubin, direct bilirubin (DBil), indirect bilirubin, globin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), glucose, body mass index (BMI), and history of diabetes (all P<0.05). The multivariate Logistic regression analysis showed that CAP (odds ratio [OR]=1.015, 95% confidence interval [CI]: 1.006 — 1.024, P=0.010), DBil (OR=1.345, 95%CI: 1.139 — 1.590, P=0.001), ALP (OR=1.019, 95%CI: 1.008 — 1.029, P=0.001), GGT (OR=1.004, 95%CI: 1.000 — 1.008, P=0.035) and BMI (OR=1.240, 95%CI: 1.137 — 1.353, P=0.001) were independent risk factors for the development of advanced liver fibrosis in NAFLD. A nomogram model was established based on the results of the multivariate Logistic regression analysis. The ROC curve analysis showed that this nomogram model had an area under the ROC curve of 0.841 (95%CI: 0.791 — 0.891) in predicting the development of advanced liver fibrosis in the NAFLD population, and the calibration curve showed a good degree of fitting between the observed and predicted values for the development of advanced liver fibrosis. Conclusion Elevated levels of CAP, BMI, DBil, ALP, and GGT are independent risk factors for advanced liver fibrosis in NAFLD. The nomogram model established based on these factors has good predictive performance and a certain value in predicting advanced liver fibrosis. -
Key words:
- Non-Alcoholic Fatty Liver Disease /
- Hepatic Fibrosis /
- Risk Factors /
- Nomograms
-
表 1 两组间一般资料比较
Table 1. Comparison of general data between two groups
指标 进展期肝纤维化组(n=65) 非进展期肝纤维化组(n=341) 统计值 P值 性别[例(%)] χ2=0.014 0.906 男 50(76.9) 260(76.2) 女 15(23.1) 81(23.8) 年龄(岁) 39.09±11.96 39.25±12.25 t=1.985 0.048 CAP(dB/m) 341(314~341) 306(272~334) Z=-4.999 0.001 TBil(μmol/L) 18.4(13.7~23.3) 14.2(10.9~18.6) Z=-3.857 0.001 DBil(μmol/L) 3.9(2.9~5.3) 2.8(2.0~4.0) Z=-4.734 0.001 IBil(μmol/L) 14.3(10.3~18.0) 11.2(8.8~14.7) Z=-3.302 0.001 Alb(g/L) 44.7(42.4~46.5) 44.4(41.9~46.9) Z=-0.438 0.661 Glb(g/L) 28.4(26.3~31.4) 27.2(25.0~29.5) Z=-2.348 0.019 ALT(U/L) 61.4(38.3~112.9) 39.4(23.6~67.2) Z=-4.238 0.001 AST(U/L) 42.2(30.7~65.3) 27.4(21.1~38.4) Z=-6.212 0.001 ALP(U/L) 86.3(72.9~106.1) 76.8(66.0~91.4) Z=-3.481 0.001 GGT(U/L) 59.5(43.3~105.1) 44.1(28.8~80.2) Z=-3.735 0.001 Cr(μmol/L) 61.5(49.9~74.8) 65.2(56.4~72.7) Z=-0.736 0.465 UA(μmol/L) 403.95(322.10~504.05) 395.80(334.50~473.90) Z=-0.627 0.530 GLU(μmol/L) 5.77(4.98~6.64) 5.28(4.85~5.80) Z=-2.852 0.040 TC(mmol/L) 5.14(4.59~5.70) 4.83(4.17~5.51) Z=-1.611 0.107 TG(mmol/L) 1.93(1.41~2.59) 1.86(1.31~2.78) Z=-0.008 0.994 HDL-C(mmol/L) 1.03(0.90~1.30) 1.11(0.95~1.29) Z=-0.905 0.366 BMI(kg/m2) 31.4(28.5~34.6) 28.1(25.4~30.7) Z=-6.061 0.001 糖尿病病史[例(%)] 15(23.1) 42(12.3) χ2=5.238 0.022 高血压病史[例(%)] 12(18.5) 64(18.8) χ2=0.003 0.954 结直肠息肉病史[例(%)] 11(16.9) 95(27.9) χ2=3.655 0.056 表 2 NAFLD患者发生进展期肝纤维化的危险因素
Table 2. Risk factors for advanced liver fibrosis in NAFLD patients
变量 B值 Wald OR 95%CI P值 CAP 0.015 3.31 1.015 1.006~1.024 0.010 DBil 0.297 3.48 1.345 1.139~1.590 0.001 ALP 0.019 3.52 1.019 1.008~1.029 0.001 GGT 0.004 2.10 1.004 1.000~1.008 0.035 BMI 0.215 4.84 1.240 1.137~1.353 0.001 -
[1] LAZARUS JV, COLOMBO M, CORTEZ-PINTO H, et al. NAFLD-sounding the alarm on a silent epidemic[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 7): 377- 379. DOI: 10.1038/s41575-020-0315-7. [2] POWELL EE, WONG VW, RINELLA M. Non-alcoholic fatty liver disease[J]. Lancet, 2021, 397( 10290): 2212- 2224. DOI: 10.1016/S0140-6736(20)32511-3. [3] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. [4] PETTA S, WONG VWS, CAMMÀ C, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values[J]. Hepatology, 2017, 65( 4): 1145- 1155. DOI: 10.1002/hep.28843. [5] MÓZES FE, LEE JA, SELVARAJ EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis[J]. Gut, 2022, 71( 5): 1006- 1019. DOI: 10.1136/gutjnl-2021-324243. [6] Liver Disease Committee, Chinese Association of Integrative Medicine. Guidelines for the diagnosis and treatment of liver fibrosis in integrative medicine practice(2019)[J]. J Clin Hepatol, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007.中国中西医结合学会肝病专业委员会. 肝纤维化中西医结合诊疗指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007. [7] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [8] SINGH S, ALLEN AM, WANG Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies[J]. Clin Gastroenterol Hepatol, 2015, 13( 4): 643- 654. DOI: 10.1016/j.cgh.2014.04.014. [9] ZHANG Y, SU XY, XIE JY, et al. An excerpt of EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update[J]. J Clin Hepatol, 2021, 37( 11): 2550- 2554. DOI: 10.3969/j.issn.1001-5256.2021.11.013.张悦, 粟兴洋, 谢静怡, 等.《欧洲肝病学会临床实践指南:评估肝脏疾病严重程度及预后的无创检测(2021年更新)》摘译[J]. 临床肝胆病杂志, 2021, 37( 11): 2550- 2554. DOI: 10.3969/j.issn.1001-5256.2021.11.013. [10] UNALP-ARIDA A, RUHL CE. Liver fibrosis scores predict liver disease mortality in the United States population[J]. Hepatology, 2017, 66( 1): 84- 95. DOI: 10.1002/hep.29113. [11] LEE J, VALI Y, BOURSIER J, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review[J]. Liver Int, 2021, 41( 2): 261- 270. DOI: 10.1111/liv.14669. [12] SHEN YX, SHI HH, LUO JL, et al. Application and progress of liver fibrosis risk prediction model for nonalcoholic fatty liver disease[J]. J Clin Med Pract, 2023, 27( 9): 131- 136, 142. DOI: 10.7619/jcmp.20222652.沈颖筱, 施惠海, 罗家乐, 等. 非酒精性脂肪性肝病肝纤维化风险预测模型的应用与进展[J]. 实用临床医药杂志, 2023, 27( 9): 131- 136, 142. DOI: 10.7619/jcmp.20222652. [13] VALI Y, LEE J, BOURSIER J, et al. FibroTest for evaluating fibrosis in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis[J]. J Clin Med, 2021, 10( 11): 2415. DOI: 10.3390/jcm10112415. [14] HARRISON SA, RATZIU V, BOURSIER J, et al. A blood-based biomarker panel(NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: A prospective derivation and global validation study[J]. Lancet Gastroenterol Hepatol, 2020, 5( 11): 970- 985. DOI: 10.1016/S2468-1253(20)30252-1. [15] DAY JW, ROSENBERG WM. The enhanced liver fibrosis(ELF) test in diagnosis and management of liver fibrosis[J]. Br J Hosp Med(Lond), 2018, 79( 12): 694- 699. DOI: 10.12968/hmed.2018.79.12.694. [16] LIU SY. Association between vitamin D deficiency and non-alcoholic fatty liver disease and liver fibrosis[D]. Fuzhou: Fujian Medical University, 2020.刘世滢. 维生素D缺乏与非酒精性脂肪性肝病及肝纤维化的相关性研究[D]. 福州: 福建医科大学, 2020. [17] NUERJIMA AHNYZ, LIU YJ, LIANG CC, et al. Correlation between the triglyceride glucose index and the degree of steatosis and liver fibrosis in nonalcoholic fatty liver disease[J]. J Hainan Med Univ, 2023, 29( 23): 1794- 1800. DOI: 10.13210/j.cnki.jhmu.20231017.002.努尔吉马·阿合尼牙孜, 刘一佳, 梁灿灿, 等. 甘油三酯葡萄糖指数与非酒精性脂肪性肝病脂肪变程度及肝纤维化的相关性研究[J]. 海南医学院学报, 2023, 29( 23): 1794- 1800. DOI: 10.13210/j.cnki.jhmu.20231017.002. [18] KIM M, YOON EL, CHO S, et al. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea[J]. Liver Int, 2022, 42( 7): 1536- 1544. DOI: 10.1111/liv.15259. [19] LIU Y, CUI SS, BAO SQ, et al. Study on metabolic-associated fatty liver disease and metabolic syndrome[J]. J Med Inf, 2022, 35( 1): 1- 5. DOI: 10.3969/j.issn.1006-1959.2022.01.001.刘英, 崔姗姗, 暴素青, 等. 代谢相关性脂肪性肝病与代谢综合征的研究[J]. 医学信息, 2022, 35( 1): 1- 5. DOI: 10.3969/j.issn.1006-1959.2022.01.001. [20] LI W, HAN YX, XIE SZ, et al. The impact of weight loss on the severity of liver fibrosis and fatty liver in patients with chronic hepatitis B and/or non-alcoholic fatty liver disease[J]. Chin Hepatol, 2023, 28( 2): 203- 206. DOI: 10.3969/j.issn.1008-1704.2023.02.015.厉绾, 韩艳霞, 谢寿珍, 等. 减重对CHB和(或)NAFLD患者肝纤维化及脂肪肝程度的影响[J]. 肝脏, 2023, 28( 2): 203- 206. DOI: 10.3969/j.issn.1008-1704.2023.02.015. [21] WANG CE, XU WT, GONG J, et al. Research progress in the treatment of non-alcoholic fatty liver disease[J]. Clin J Med Offic, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06.王彩娥, 许文涛, 宫建, 等. 非酒精性脂肪性肝病治疗研究进展[J]. 临床军医杂志, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06. [22] SORRENTINO P, TARANTINO G, PERRELLA A, et al. A clinical-morphological study on cholestatic presentation of nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2005, 50( 6): 1130- 1135. DOI: 10.1007/s10620-005-2719-1. [23] CHEN RL, MA X. Pathogenesis of cholestasis-induced liver fibrosis and thoughts for blockade[J]. J Clin Hepatol, 2019, 35( 2): 247- 251. DOI: 10.3969/j.issn.1001-5256.2019.02.002.陈瑞玲, 马雄. 胆汁淤积导致肝纤维化的机制及其阻断策略[J]. 临床肝胆病杂志, 2019, 35( 2): 247- 251. DOI: 10.3969/j.issn.1001-5256.2019.02.002. -



 PDF下载 ( 1014 KB)
PDF下载 ( 1014 KB)

 下载:
下载: