酪氨酸激酶抑制剂联合免疫检查点抑制剂在中晚期肝细胞癌二线治疗中的效果及安全性分析
DOI: 10.12449/JCH240818
Efficacy and safety of tyrosine kinase inhibitor combined with immune checkpoint inhibitor as the second-line therapy for advanced hepatocellular carcinoma
-
摘要:
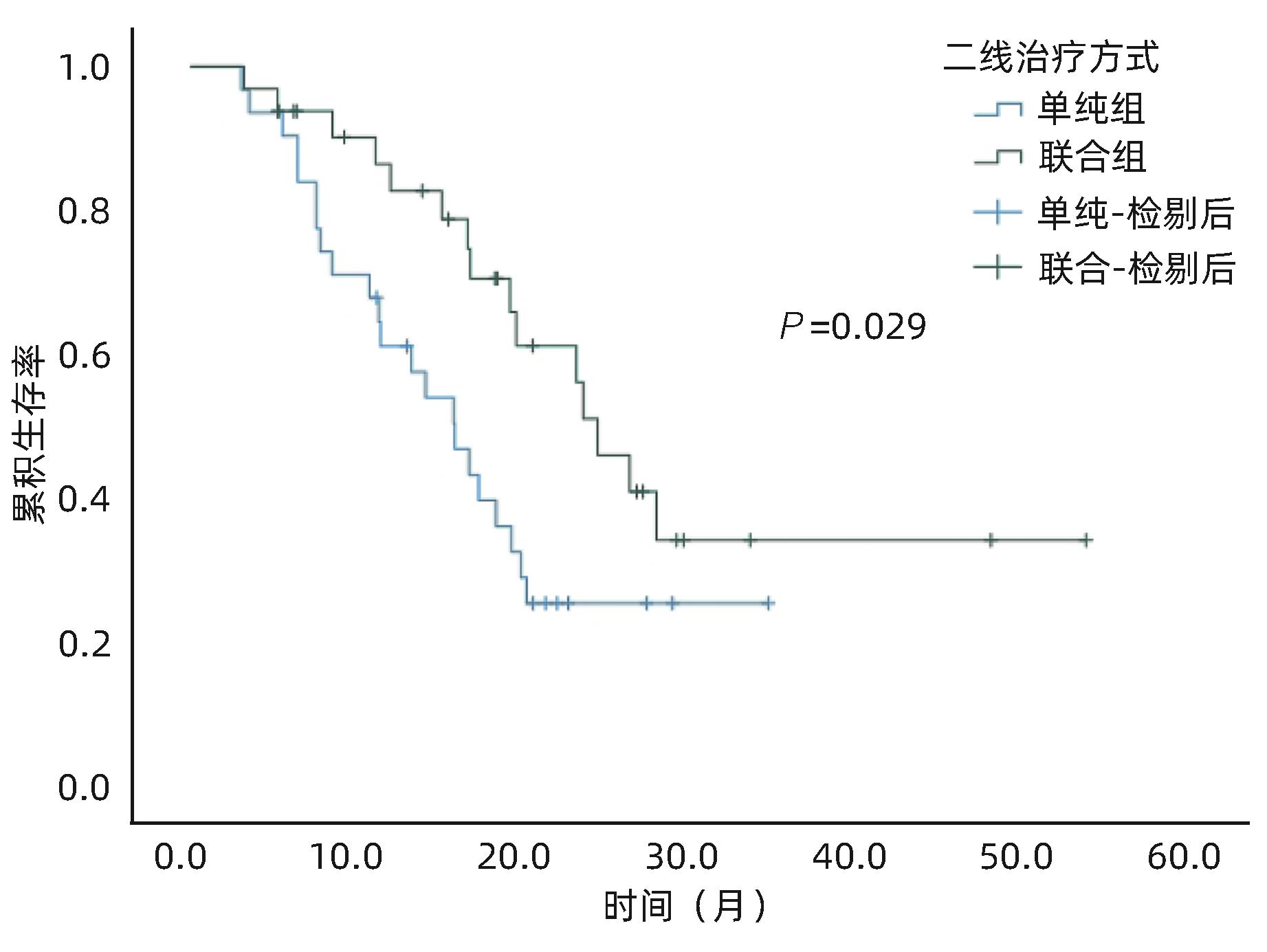
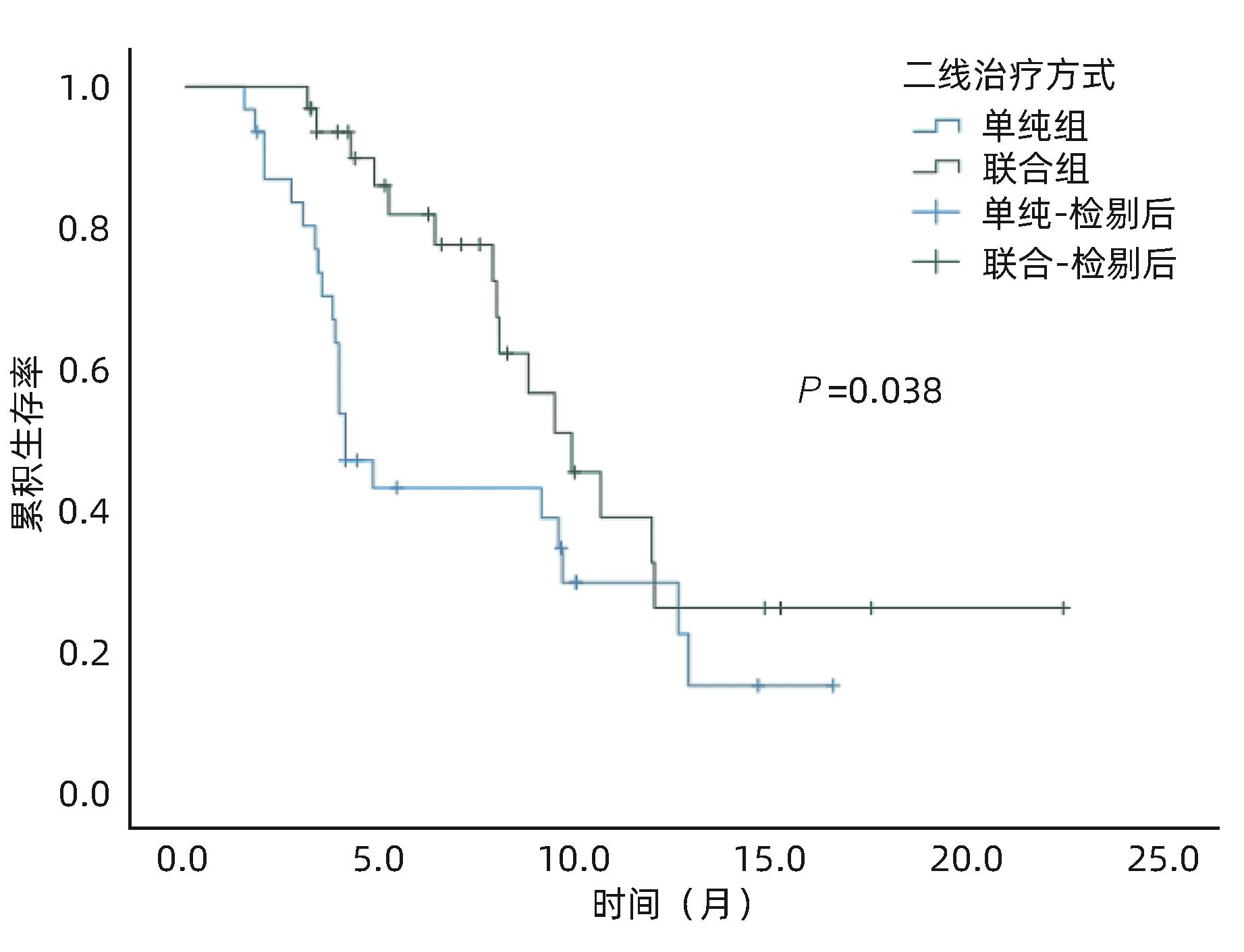
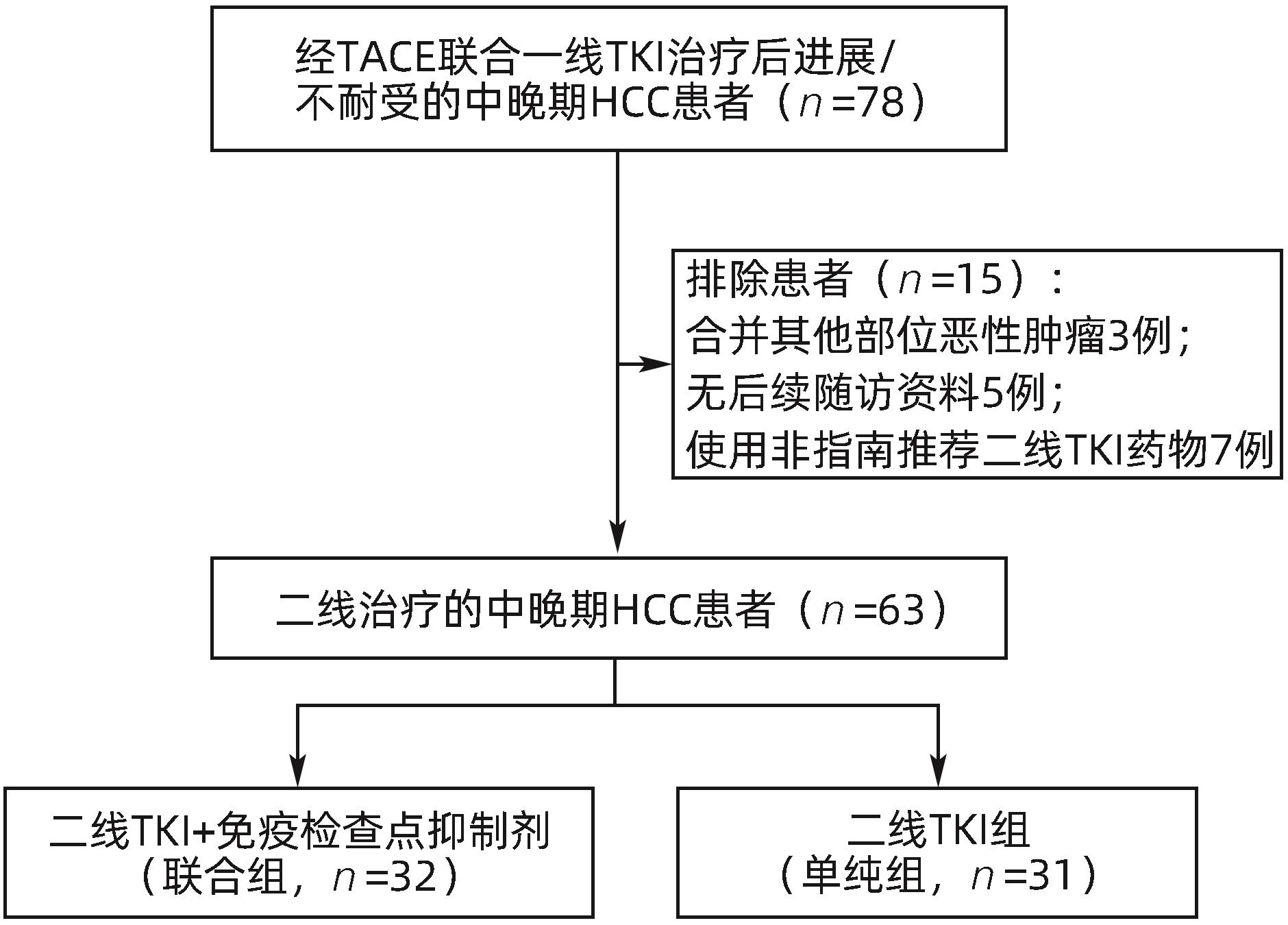
目的 评估酪氨酸激酶抑制剂(TKI)联合免疫检查点抑制剂在中晚期肝细胞癌(HCC)二线治疗中的效果及安全性。 方法 收集2018年1月—2022年12月苏州大学附属第一医院介入科收治的经过经肝动脉化疗栓塞术(TACE)联合一线TKI治疗后进展/不耐受,换用二线TKI联合或者不联合免疫检查点抑制剂的63例中晚期HCC患者的临床资料。其中使用二线TKI联合免疫检查点抑制剂共32例(联合组),使用二线TKI共31例(单纯组)。依据改良实体瘤疗效评价标准(mRECIST)评估肿瘤反应,根据通用不良事件术语5.0标准(CTCAE 5.0)评估不良事件。使用Kaplan-Meier法计算两组的中位生存期(mOS)、中位无进展生存期(mPFS)。比较两组的客观缓解率(ORR)和疾病控制率(DCR)。基线资料及随访结果的组间比较采用χ2检验。 结果 63例患者中位随访时间为16.5(3.2~53.4)个月。联合组mOS、mPFS 分别为24.3个月(95%CI:20.0~28.6)和9.8个月(95%CI:7.5~12.1),15.8个月(95%CI:11.4~20.1)和4.1个月(95%CI:3.2~4.9),两组mOS、mPFS之间差异均有统计学意义(P值分别为0.029、0.038)。联合组ORR和DCR分别为47%和84%,单纯组为19%和65%,两组之间ORR差异有统计学意义(P=0.021),DCR差异无统计学意义(P=0.070)。联合组中4例(12.5%)、单纯组中3例(10.0%)发生Ⅲ~Ⅳ级严重不良事件,两组均无药物不良反应致死事件,两组患者不良事件的发生率比较,差异无统计学意义(P=0.783)。 结论 与单用TKI治疗相比,TKI联合免疫检查点抑制剂作为中晚期HCC二线治疗的疗效更显著,且不会增加严重不良反应。 Abstract:Objective To investigate the efficacy and safety of tyrosine kinase inhibitor (TKI) combined with immune checkpoint inhibitor as the second-line therapy for advanced hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for the clinical data of 63 patients with advanced HCC who were admitted to Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, from January 2018 to December 2022, and all patients experienced progression/intolerance after transcatheter arterial chemoembolization combined with first-line TKI and were switched to second-line TKI with or without immune checkpoint inhibitor. The 32 patients receiving second-line TKI with immune checkpoint inhibitor were enrolled as combination group, and the 31 patients receiving second-line TKI alone were enrolled as single treatment group. Modified Response Evaluation Criteria in Solid Tumors was used to evaluate tumor response, and Common Terminology Criteria for Adverse Events 5.0 was used to evaluate adverse events. The Kaplan-Meier method was used to calculate median overall survival (mOS) and median progression-free survival (mPFS) for the two groups, and the two groups were compared in terms of objective response rate (ORR) and disease control rate (DCR). The chi-square test was used for comparison of baseline data and follow-up results between groups. Results The median follow-up time was 16.5 (3.2 — 53.4) months for the 63 patients. The combination group had an mOS of 24.3 (95% confidence interval [CI]: 20.0 — 28.6) months and an mPFS of 9.8 (95%CI: 7.5 — 12.1) months, while the single treatment group had an mOS of 15.8 (95%CI: 11.4 — 20.1) months and an mPFS of 4.1 (95%CI: 3.2 — 4.9) months, and there were significant differences in mOS and mPFS between the two groups (P=0.029 and 0.038). The combination group had an ORR of 47% and a DCR of 84%, while the single treatment group had an ORR of 19% and a DCR of 65%; there was a significant difference in ORR between the two groups (P=0.021), but with no significant difference in DCR between the two groups (P=0.070). As for adverse events, 4 patients (12.5%) in the combination group and 3 (10.0%) in the single treatment group experienced grade Ⅲ — Ⅳ serious adverse events, with no fatal drug reactions in either group, and there was no significant difference in the incidence rate of adverse events between the two groups (P=0.783). Conclusion Compared with TKI alone, TKI combined with immune checkpoint inhibitor has a more significant therapeutic effect as the second-line therapy for advanced HCC, without increasing serious adverse reactions. -
表 1 两组患者基线资料比较
Table 1. Baseline characteristics of two groups
指标 单纯组 (n=31) 联合组 (n=32) P值 年龄[例(%)] 0.513 ≤60岁 12(38.7) 15(46.9) >60岁 19(61.3) 17(53.1) 性别[例(%)] 0.143 男 29(93.5) 26(81.3) 女 2(6.5) 6(18.7) 病因[例(%)] 0.179 乙型肝炎 23(74.2) 28(87.5) 其他 8(25.8) 4(12.5) 病灶个数[例(%)] 0.373 ≤3个 12(38.7) 9(28.1) >3个 19(61.3) 23(71.9) 血管侵犯[例(%)] 0.898 无 16(51.6) 16(50.0) 有 15(48.4) 16(50.0) 病灶大小[例(%)] 0.513 ≤5 cm 12(38.7) 15(46.9) >5 cm 19(61.3) 17(53.1) 肝外转移[例(%)] 0.338 无 22(71.0) 26(81.3) 有 9(29.0) 6(18.7) Child-Pugh分级[例(%)] 0.910 A级 17(54.8) 18(56.2) B级 14(45.2) 14(43.8) ALBI评分[例(%)] 0.563 Ⅰ 6(19.4) 7(21.9) Ⅱ 24(77.4) 22(68.7) Ⅲ 1(3.2) 3(9.4) ECOG评分[例(%)] 0.649 0 9(29.0) 11(34.4) 1 22(71.0) 21(65.6) AFP[例(%)] 0.932 ≤400 ng/mL 10(32.3) 10(31.2) >400 ng/mL 21(67.7) 22(68.8) 外科切除史[例(%)] 0.153 无 10(32.3) 16(50.0) 有 21(67.7) 16(50.0) 二线是否有过TACE[例(%)] 0.722 无 11(35.5) 10(31.3) 有 20(64.5) 22(68.7) 一线治疗方案[例(%)] 0.261 TACE+仑伐替尼 18(58.1) 14(43.8) TACE+索拉非尼 12(38.7) 18(56.2) TACE+多纳非尼 1(3.2) 0(0.0) 表 2 两组疗效对比
Table 2. Treatment responses in combination group and control group
指标 单纯组(n=31) 联合组(n=32) CR(例) 1 3 PR(例) 5 12 SD(例) 14 12 PD(例) 11 5 ORR(%) 19 47 DCR(%) 65 84 表 3 两组不良事件发生情况对比
Table 3. Adverse events in the two groups
不良反应 单纯组(n=31) 联合组(n=32) Ⅰ~Ⅱ级 Ⅲ~Ⅳ级 Ⅰ~Ⅱ级 Ⅲ~Ⅳ级 肝功能损伤(例) 27 1 26 1 手足反应(例) 25 1 26 0 高血压(例) 23 0 19 0 发热(例) 13 0 17 0 蛋白尿(例) 7 1 5 0 消化道症状(例) 22 0 24 0 腹痛(例) 11 0 16 0 免疫相关性肺炎(例) 0 0 2 0 免疫相关性心肌炎(例) 0 0 1 1 免疫相关性肠炎(例) 0 0 1 1 免疫相关性甲减(例) 0 0 1 1 -
[1] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [2] Clinical Guidelines Committee of Chinese College of Interventionalists. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma(2023 edition)[J]. Natl Med J China, 2023, 103( 34): 2674- 2694. DOI: 10.3760/cma.j.cn112137-20230630-01114.中国医师协会介入医师分会临床诊疗指南专委会. 中国肝细胞癌经动脉化疗栓塞(TACE)治疗临床实践指南(2023年版)[J]. 中华医学杂志, 2023, 103( 34): 2674- 2694. DOI: 10.3760/cma.j.cn112137-20230630-01114. [3] CHIU DK, TSE AP, XU IM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma[J]. Nat Commun, 2017, 8( 1): 517. DOI: 10.1038/s41467-017-00530-7. [4] XING R, GAO J, CUI Q, et al. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma[J]. Front Immunol, 2021, 12: 783236. DOI: 10.3389/fimmu.2021.783236. [5] LAFACE C, FEDELE P, MASELLI FM, et al. Targeted therapy for hepatocellular carcinoma: old and new opportunities[J]. Cancers(Basel), 2022, 14( 16): 4028. DOI: 10.3390/cancers14164028. [6] GALLE PR, FINN RS, QIN S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma(IMbrave150): an open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2021, 22( 7): 991- 1001. DOI: 10.1016/S1470-2045(21)00151-0. [7] REN Z, XU J, BAI Y, et al. Sintilimab plus a bevacizumab biosimilar(IBI305) versus sorafenib in unresectable hepatocellular carcinoma(ORIENT-32): a randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22( 7): 977- 990. DOI: 10.1016/S1470-2045(21)00252-7. [8] QIN S, CHAN SL, GU S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma(CARES-310): a randomised, open-label, international phase 3 study[J]. Lancet, 2023, 402( 10408): 1133- 1146. DOI: 10.1016/S0140-6736(23)00961-3. [9] KUDO M. Atezolizumab plus bevacizumab followed by curative conversion(ABC Conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma[J]. Liver Cancer, 2022, 11( 5): 399- 406. DOI: 10.1159/000526163. [10] KUDO M, HAN KH, YE SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements[J]. Liver Cancer, 2020, 9( 3): 245- 260. DOI: 10.1159/000507370. [11] PENG Z, FAN W, ZHU B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase iii, randomized clinical trial(LAUNCH)[J]. J Clin Oncol, 2023, 41( 1): 117- 127. DOI: 10.1200/JCO.22.00392. [12] ZHU HD, LI HL, HUANG MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma(CHANCE001)[J]. Signal Transduct Target Ther, 2023, 8( 1): 58. DOI: 10.1038/s41392-022-01235-0. [13] SU GL, ALTAYAR O, O’SHEA R, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma[J]. Gastroenterology, 2022, 162( 3): 920- 934. DOI: 10.1053/j.gastro.2021.12.276. [14] BAO MH, WONG CC. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer[J]. Cells, 2021, 10( 7). DOI: 10.3390/cells10071715. [15] Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy in hepatocellular carcinoma(2021 edition)[J]. Chin J Dig Surg, 2021, 20( 6): 600- 616. DOI: 10.3760/cma.j.cn115610-20210512-00223.中国抗癌协会肝癌专业委员会转化治疗协作组. 肝癌转化治疗中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20( 6): 600- 616. DOI: 10.3760/cma.j.cn115610-20210512-00223. [16] Clinical Guidelines Committee of Chinese College of Interventionalists. Expert consensus on the TACE failure/refractoriness and its subsequent therapies in treating patients with hepatocellular carcinoma[J]. J Intervent Radiol, 2022, 31( 11): 1039- 1044. DOI: 10.3969/j.issn.1008-794X.2022.11.001.中国医师协会介入医师分会临床诊疗指南专委会. 肝细胞癌经动脉化疗栓塞抵抗及后续治疗专家共识[J]. 介入放射学杂志, 2022, 31( 11): 1039- 1044. DOI: 10.3969/j.issn.1008-794X.2022.11.001. [17] SONG YJ, QIAO JC, SONG JH. Right hepatectomy of CNLC stage IIIa hepatocellular carcinoma after successful conversion by TACE combined with targeted therapy and immunotherapy: a case report[J/OL]. Chin J Hepat Surg(Electronic Edition), 2024, 13( 2): 227- 230. DOI: 10.3877/cma.j.issn.2095-3232.2024.02.018.宋燕京, 乔江春, 宋京海. 中晚期肝癌TACE联合免疫靶向转化治疗后右半肝切除术一例[J/OL]. 中华肝脏外科手术学电子杂志, 2024, 13( 2): 227- 230. DOI: 10.3877/cma.j.issn.2095-3232.2024.02.018. [18] CHENG YR, YAN D, YANG JD, et al. Effect of hepatic artery embolization chemotherapy combined with sorafenib in the treatment of primary liver cancer and its impact on patient immune function[J]. Clin J Med Offic, 2021, 49( 3): 290- 291. DOI: 10.16680/j.1671-3826.2021.03.16.程瑜蓉, 严冬, 杨建东, 等. 肝动脉栓塞化疗联合索拉非尼在原发性肝癌治疗中应用效果及对患者免疫功能影响[J]. 临床军医杂志, 2021, 49( 3): 290- 291. DOI: 10.16680/j.1671-3826.2021.03.16. [19] BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment(RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389( 10064): 56- 66. DOI: 10.1016/S0140-6736(16)32453-9. [20] QIN S, LI Q, GU S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma(AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6( 7): 559- 568. DOI: 10.1016/S2468-1253(21)00109-6. [21] XU Y, FU S, SHANG K, et al. PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus regorafenib in patients with advanced hepatocellular carcinoma after failure of sorafenib[J]. Front Oncol, 2022, 12: 958869. DOI: 10.3389/fonc.2022.958869. [22] HUANG J, GUO Y, HUANG W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study[J]. J Hepatocell Carcinoma, 2022, 9: 157- 170. DOI: 10.2147/JHC.S353956. [23] LIU J, TAO H, YUAN T, et al. Immunomodulatory effects of regorafenib: Enhancing the efficacy of anti-PD-1/PD-L1 therapy[J]. Front Immunol, 2022, 13: 992611. DOI: 10.3389/fimmu.2022.992611. [24] YANG Y, WANG C, SUN H, et al. Apatinib prevents natural killer cell dysfunction to enhance the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma[J]. Cancer Gene Ther, 2021, 28( 1-2): 89- 97. DOI: 10.1038/s41417-020-0186-7. [25] ZOU X, XU Q, YOU R, et al. Efficacy and safety of TACE combined with regorafenib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma after sorafenib resistance[J]. J Hepatocell Carcinoma, 2023, 10: 267- 279. DOI: 10.2147/JHC.S399874. [26] CAO Y, OUYANG T, XIONG F, et al. Efficacy of apatinib in patients with sorafenib-transarterial chemoembolization refractory hepatocellular carcinoma: a retrospective study[J]. Hepatol Int, 2021, 15( 5): 1268- 1277. DOI: 10.1007/s12072-021-10198-3. -



 PDF下载 ( 1031 KB)
PDF下载 ( 1031 KB)

 下载:
下载: