胰腺癌类器官模型的构建及其对化疗药物的敏感性试验
DOI: 10.12449/JCH240921
Construction of pancreatic cancer organoids and their sensitivity to chemotherapy drugs
-
摘要:
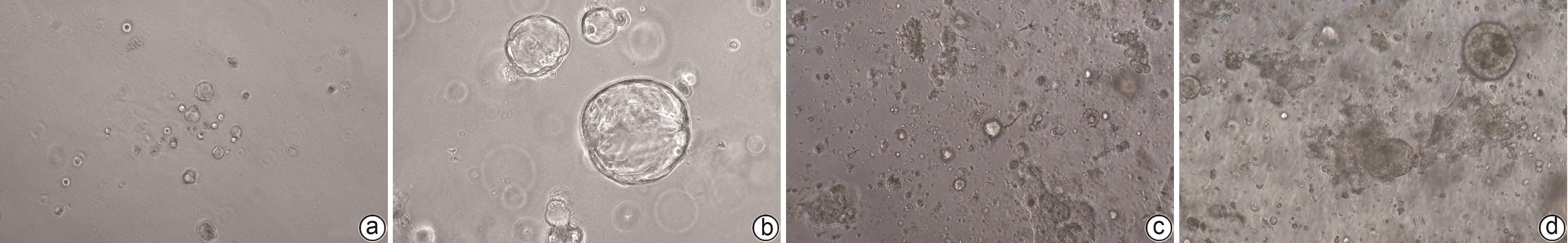
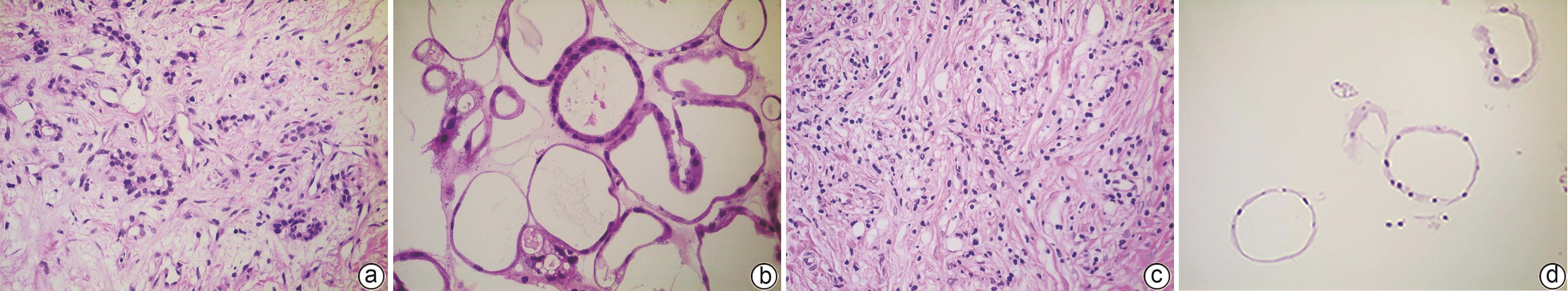
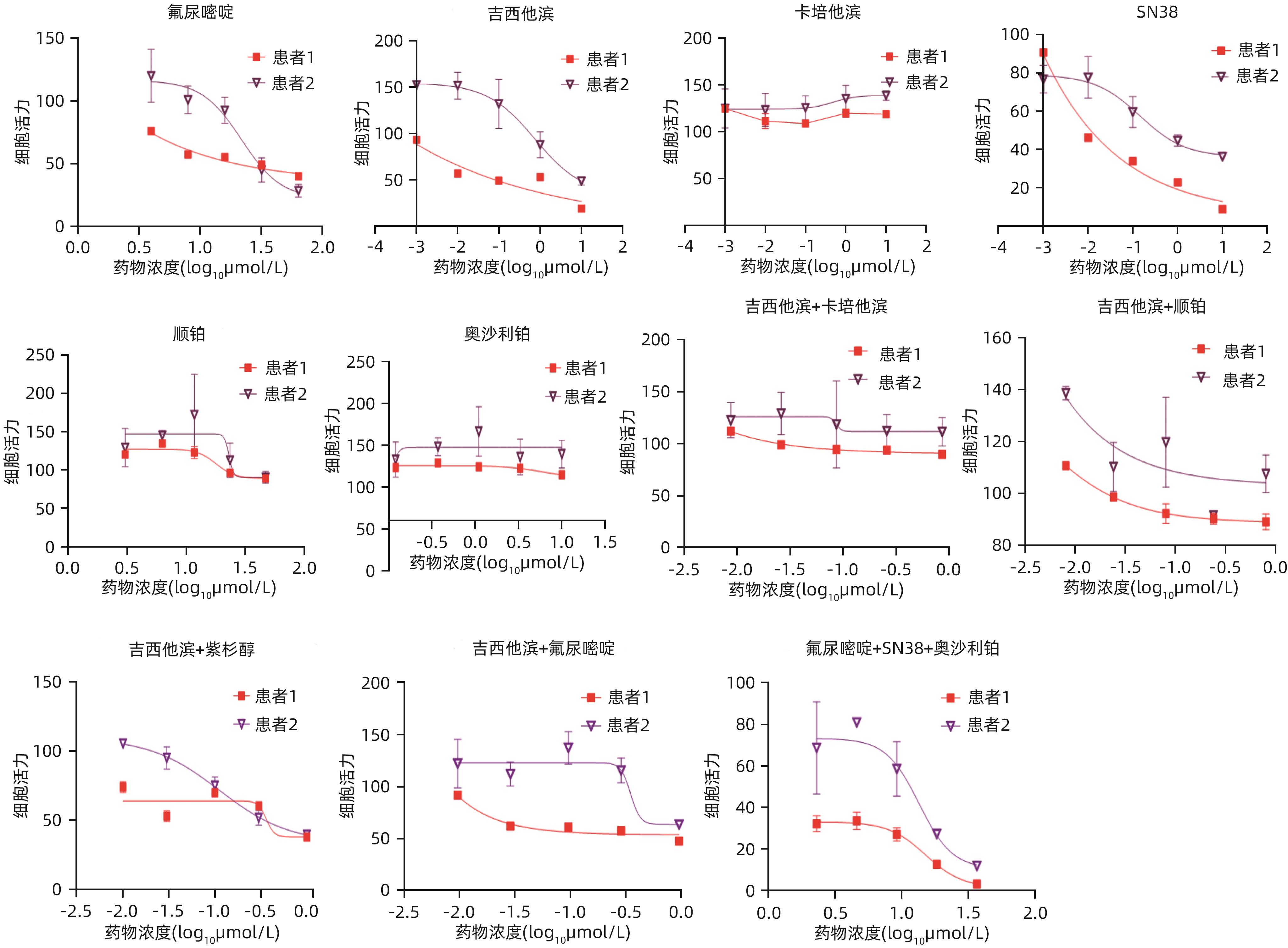
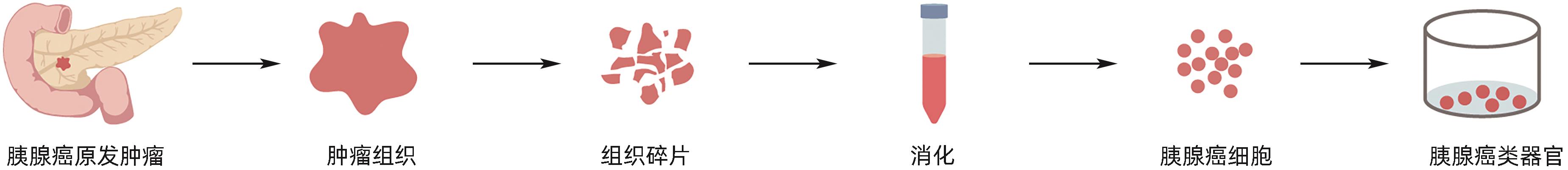
目的 建立及鉴定患者来源的类器官模型,并利用该模型进行化疗药物敏感性检测。 方法 利用已确诊胰腺癌的2例女性患者的手术标本获取肿瘤组织消化后获取胰腺癌细胞,利用基质胶接种于培养皿中进行三维培养;制备石蜡切片并进行苏木精-伊红(HE)染色和免疫组化染色,通过与亲本肿瘤组织对比,检测其能否保留体内肿瘤的组织病理学特征;利用不同浓度的7种化疗药物处理胰腺癌类器官,使用Cell Titer-Glo®3D试剂测定细胞活力,分析药敏结果。 结果 成功建立了2例患者来源的胰腺癌类器官,HE染色和免疫组化染色结果显示胰腺癌类器官与其来源的患者肿瘤在组织病理学特征上一致;2例胰腺癌类器官均对吉西他滨单药、奥沙利铂与SN38+氟尿嘧啶联用更为敏感,患者1较患者2敏感性更高,来自不同患者的类器官对药物反应存在个体差异。 结论 本研究成功构建的胰腺癌类器官模型能够反映亲本胰腺肿瘤的组织学分型,并能够进行体外化疗药物敏感性试验,有望为患者临床用药提供参考。 Abstract:Objective To construct and identify a patient-derived organoid model, and to investigate the sensitivity of chemotherapy drugs using this model. Methods Pancreatic cancer cells were obtained from the surgical specimens of two female patients with a confirmed diagnosis of pancreatic cancer after tumor tissue digestion, and then the cells were inoculated into a culture dish using matrigel for three-dimensional culture. Paraffin sections were prepared for HE staining and immunohistochemical staining and were compared with the parent tumor tissue to determine whether the histopathological features of the tumor in vivo were preserved. The pancreatic cancer organoids were treated with seven chemotherapy drugs at different concentrations; Cell Titer-Glo®3D reagent was used to measure cell viability, and the results of drug sensitivity were analyzed. Results Two patient-derived pancreatic cancer organoids were successfully constructed, and HE staining and immunohistochemical staining showed that the pancreatic cancer organoids had consistent histopathological features with the tumors of the corresponding patient. Both pancreatic cancer organoids were more sensitive to gemcitabine monotherapy and the combination of oxaliplatin+SN38+fluorouracil, and patient 1 was more sensitive than patient 2. There were individual differences in the response to drugs between the organoids from different patients. Conclusion The pancreatic cancer organoid model successfully constructed in this study can reflect the histological classification of parent pancreatic tumors and can be used for in vitro chemotherapy drug sensitivity test, which is expected to provide a reference for clinical medication. -
Key words:
- Pancreatic Neoplasms /
- Organoids /
- Drug Therapy, Combination /
- Sensitivity Tests
-
表 1 药物的具体浓度
Table 1. The specific concentration of the drug
药物名称 梯度1 梯度2 梯度3 梯度4 梯度5 梯度6 氟尿嘧啶 64 μmol/L 32 μmol/L 16 μmol/L 8 μmol/L 4 μmol/L 0 吉西他滨 10 μmol/L 1 μmol/L 100 nmol/L 10 nmol/L 1 nmol/L 0 卡培他滨 10 μmol/L 1 μmol/L 100 nmol/L 10 nmol/L 1 nmol/L 0 SN38 10 μmol/L 1 μmol/L 100 nmol/L 10 nmol/L 1 nmol/L 0 顺铂 50 μmol/L 25 μmol/L 12.5 μmol/L 6.25 μmol/L 3.125 μmol/L 0 奥沙利铂 10 μmol/L 3.3 μmol/L 1.1 μmol/L 370 nmol/L 123 nmol/L 0 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [2] ZHU B, WU XM, GUO TY, et al. Epidemiological characteristics of pancreatic cancer in China from 1990 to 2019[J]. Cancer Control, 2021, 28: 10732748211051536. DOI: 10.1177/10732748211051536. [3] GORAL V. Pancreatic cancer: Pathogenesis and diagnosis[J]. Asian Pac J Cancer Prev, 2015, 16( 14): 5619- 5624. DOI: 10.7314/apjcp.2015.16.14.5619. [4] ZHANG LL, SANAGAPALLI S, STOITA A. Challenges in diagnosis of pancreatic cancer[J]. World J Gastroenterol, 2018, 24( 19): 2047- 2060. DOI: 10.3748/wjg.v24.i19.2047. [5] LIU KD, GENG YD, WANG LZ, et al. Systematic exploration of the underlying mechanism of gemcitabine resistance in pancreatic adenocarcinoma[J]. Mol Oncol, 2022, 16( 16): 3034- 3051. DOI: 10.1002/1878-0261.13279. [6] SYED AR, CARLETON NM, HORNE Z, et al. Survival trends for resectable pancreatic cancer using a multidisciplinary conference: The impact of post-operative chemotherapy[J]. J Gastrointest Cancer, 2020, 51( 3): 836- 843. DOI: 10.1007/s12029-019-00303-z. [7] WU S, REN JQ, WU HX, et al. Drug resistance factors in postoperative gemcitabine chemotherapy after radical resection of pancreatic cancer[J]. Chin J Dig Surg, 2023, 22( 5): 616- 622. DOI: 10.3760/cma.j.cn115610-20230323-00125.武帅, 任加强, 吴含雪, 等. 胰腺癌根治性切除术后吉西他滨化疗方案耐药因素分析[J]. 中华消化外科杂志, 2023, 22( 5): 616- 622. DOI: 10.3760/cma.j.cn115610-20230323-00125. [8] ILIC M, ILIC I. Epidemiology of pancreatic cancer[J]. World J Gastroenterol, 2016, 22( 44): 9694- 9705. DOI: 10.3748/wjg.v22.i44.9694. [9] SATO T, VRIES RG, SNIPPERT HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature, 2009, 459( 7244): 262- 265. DOI: 10.1038/nature07935. [10] SATO T, STANGE DE, FERRANTE M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium[J]. Gastroenterology, 2011, 141( 5): 1762- 1772. DOI: 10.1053/j.gastro.2011.07.050. [11] BARKER N, HUCH M, KUJALA P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro[J]. Cell Stem Cell, 2010, 6( 1): 25- 36. DOI: 10.1016/j.stem.2009.11.013. [12] BARTFELD S, BAYRAM T, VAN DE WETERING M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection[J]. Gastroenterology, 2015, 148( 1): 126- 136. e 6. DOI: 10.1053/j.gastro.2014.09.042. [13] HUCH M, GEHART H, VAN BOXTEL R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver[J]. Cell, 2015, 160( 1-2): 299- 312. DOI: 10.1016/j.cell.2014.11.050. [14] General Office of National Health Commission. Standard for diagnosis and treatment of pancreatic cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 5): 1006- 1030. DOI: 10.3969/j.issn.1001-5256.2022.05.007.国家卫生健康委办公厅. 胰腺癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 5): 1006- 1030. DOI: 10.3969/j.issn.1001-5256.2022.05.007. [15] ZHAO B. Application prospects of organoids in organ transplantation[J]. Organ Transplantation, 2022, 13( 2): 169- 175. DOI: 10.3969/j.issn.1674-7445.2022.02.004.赵冰. 类器官在器官移植领域的应用前景[J]. 器官移植, 2022, 13( 2): 169- 175. DOI: 10.3969/j.issn.1674-7445.2022.02.004. [16] SI WXR, JIANG M. Application of organoids in basic research and clinical translation of cancers[J]. Tianjin Med J, 2024, 52( 1): 28- 32. DOI: 10.11958/20231475.司吴雪蓉, 蒋明. 类器官在癌症基础研究和临床转化中的应用[J]. 天津医药, 2024, 52( 1): 28- 32. DOI: 10.11958/20231475. [17] SUN GC, LI HY, CHEN J, et al. Research progress and application of organoid in biomedicine[J]. Clin J Med Off, 2023, 51( 11): 1206- 1210. DOI: 10.16680/j.1671-3826.2023.11.28.孙广晨, 李宏宇, 陈江, 等. 类器官在生物医学中研究进展及应用[J]. 临床军医杂志, 2023, 51( 11): 1206- 1210. DOI: 10.16680/j.1671-3826.2023.11.28. [18] DEMYAN L, HABOWSKI AN, PLENKER D, et al. Pancreatic cancer patient-derived organoids can predict response to neoadjuvant chemotherapy[J]. Ann Surg, 2022, 276( 3): 450- 462. DOI: 10.1097/SLA.0000000000005558. [19] BOJ SF, HWANG CI, BAKER LA, et al. Organoid models of human and mouse ductal pancreatic cancer[J]. Cell, 2015, 160( 1-2): 324- 338. DOI: 10.1016/j.cell.2014.12.021. [20] HUCH M, BONFANTI P, BOJ SF, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis[J]. EMBO J, 2013, 32( 20): 2708- 2721. DOI: 10.1038/emboj.2013.204. [21] NEAL JT, LI XN, ZHU JJ, et al. Organoid modeling of the tumor immune microenvironment[J]. Cell, 2018, 175( 7): 1972- 1988. e 16. DOI: 10.1016/j.cell.2018.11.021. [22] SCHUTH S, BLANC SL, KRIEGER TG, et al. Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system[J]. J Exp Clin Cancer Res, 2022, 41( 1): 312. DOI: 10.1186/s13046-022-02519-7. [23] CATTANEO CM, DIJKSTRA KK, FANCHI LF, et al. Tumor organoid-T-cell coculture systems[J]. Nat Protoc, 2020, 15( 1): 15- 39. DOI: 10.1038/s41596-019-0232-9. [24] ZHANG J, TAVAKOLI H, MA L, et al. Immunotherapy discovery on tumor organoid-on-a-chip platforms that recapitulate the tumor microenvironment[J]. Adv Drug Deliv Rev, 2022, 187: 114365. DOI: 10.1016/j.addr.2022.114365. -



 PDF下载 ( 3800 KB)
PDF下载 ( 3800 KB)

 下载:
下载: