术前ALT/AST联合多期CT影像学指标对胰十二指肠切除术后临床相关胰瘘的预测价值
DOI: 10.12449/JCH240922
Value of preoperative alanine aminotransferase/aspartate aminotransferase combined with multi-phase CT radiological indicators in predicting clinically relevant pancreatic fistula after pancreaticoduodenectomy
-
摘要:
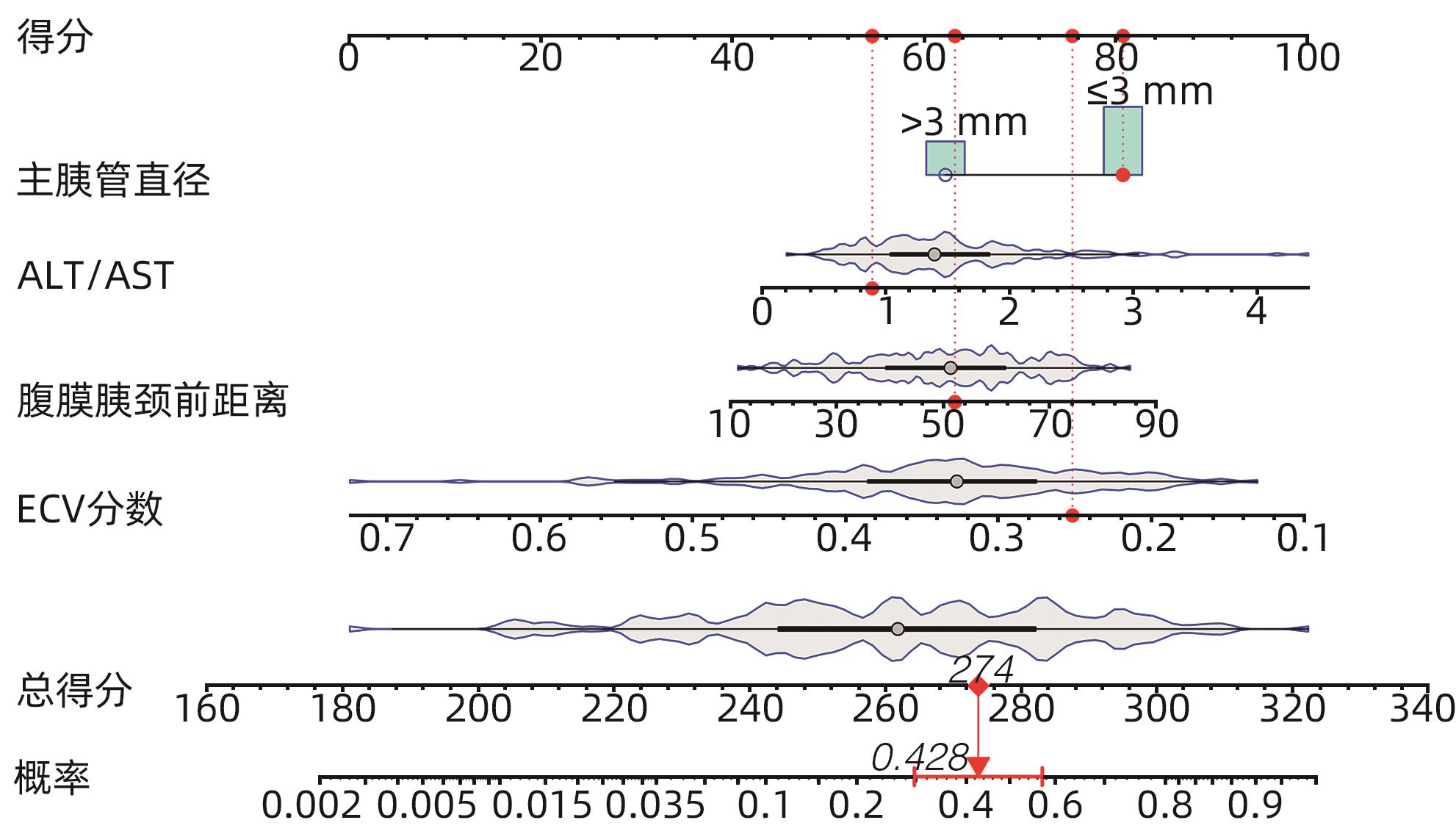
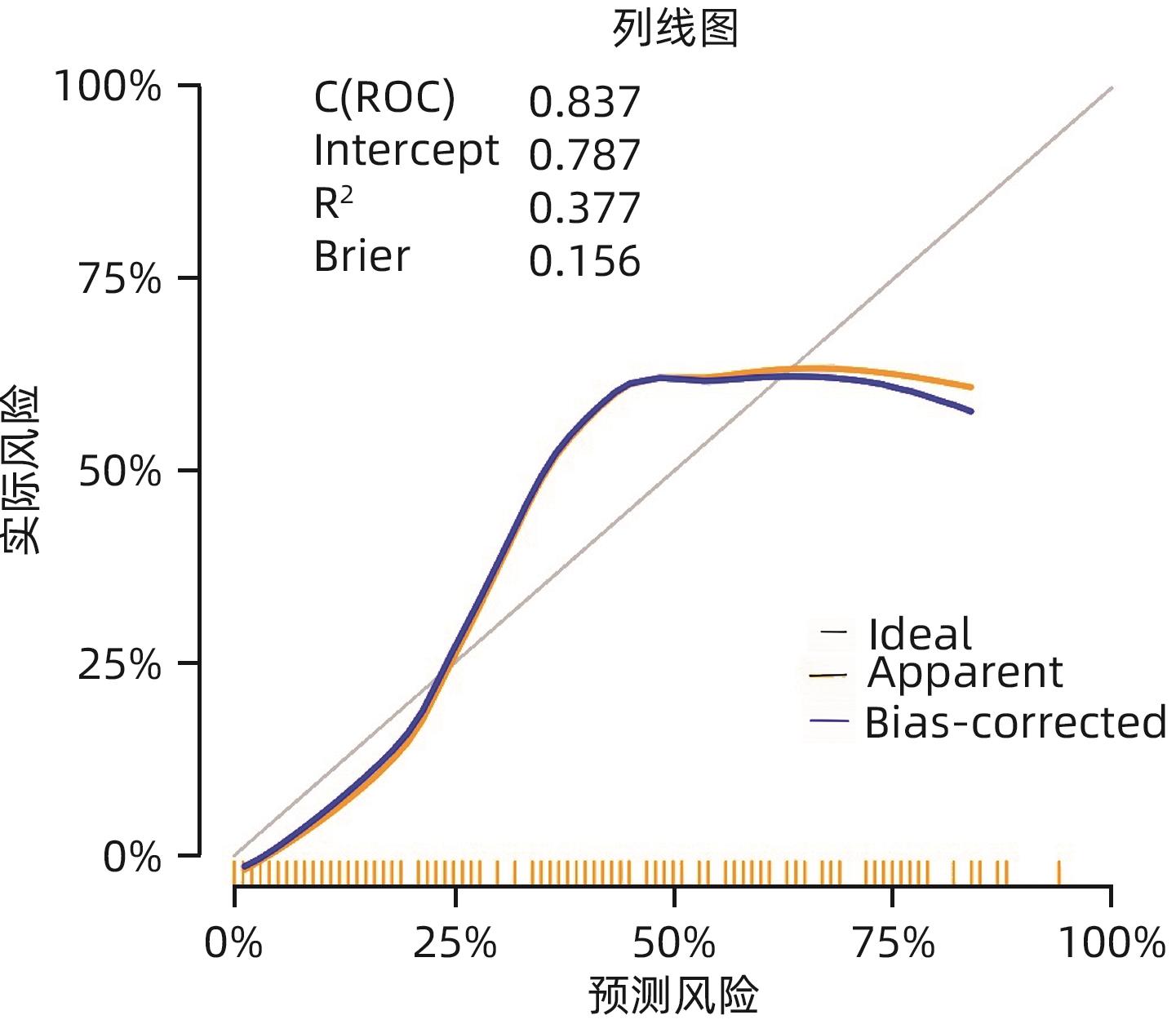
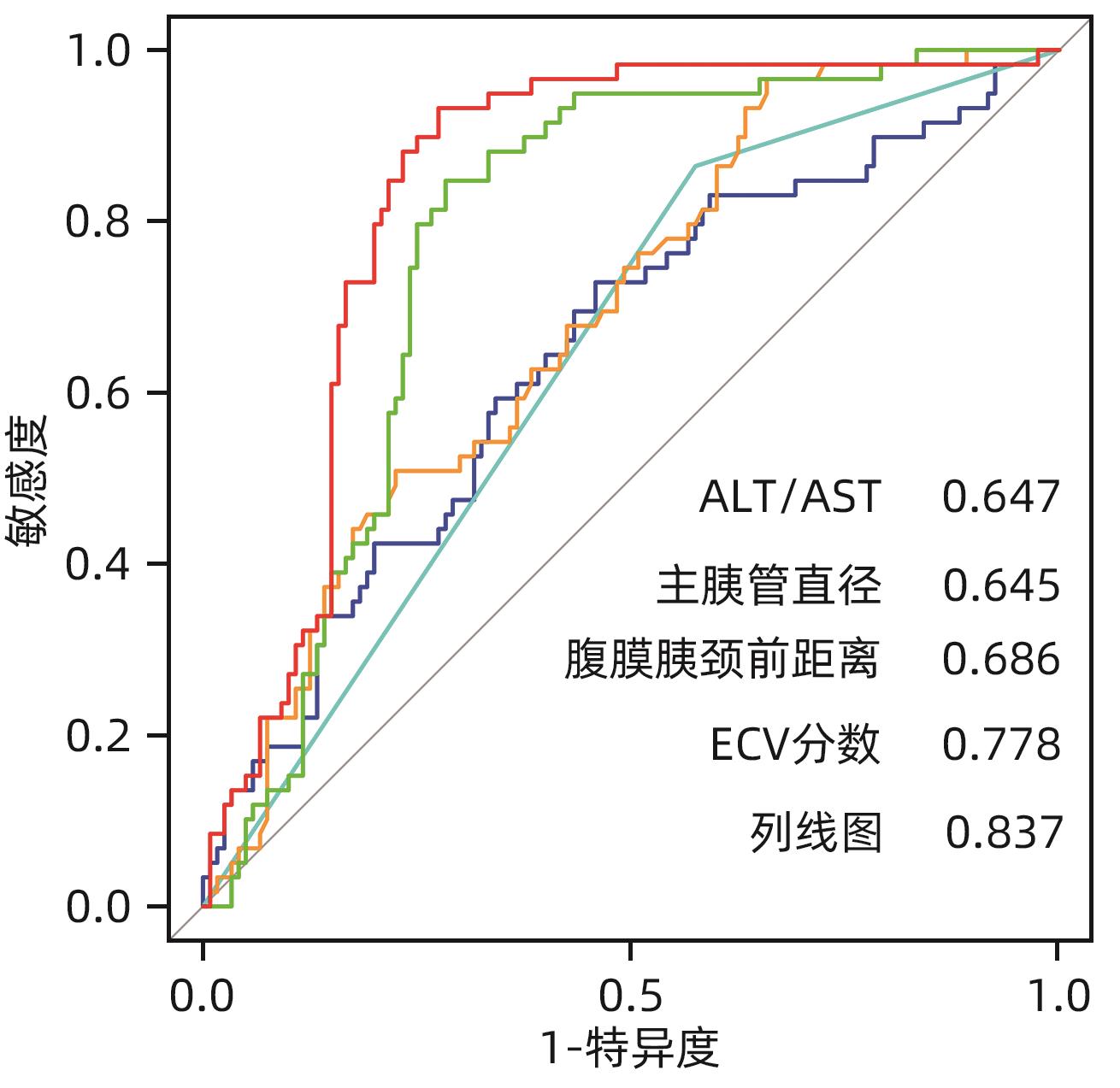
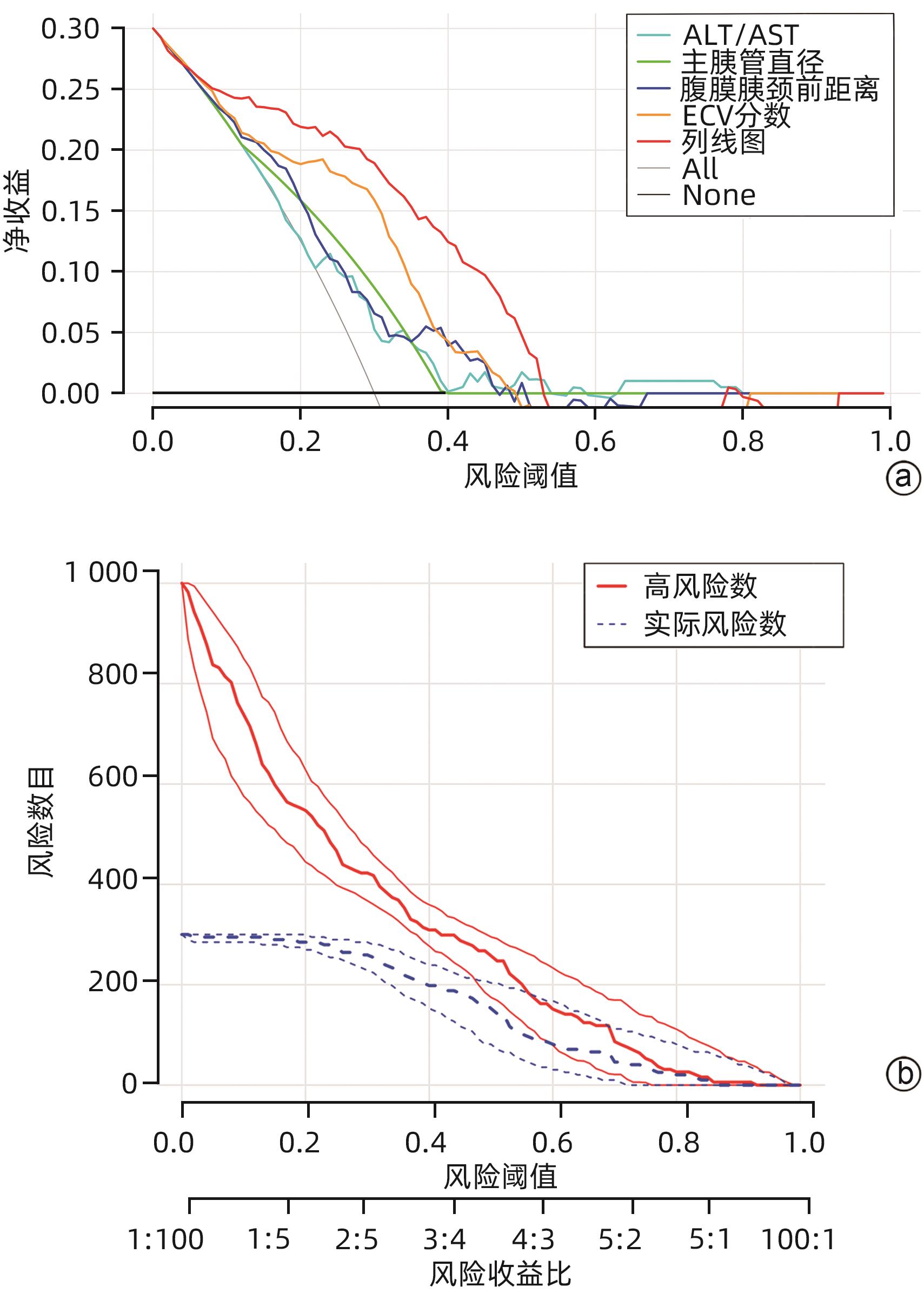
目的 探讨胰十二指肠切除术后发生临床相关胰瘘(CR-POPF)的危险因素,并建立预测模型,对CR-POPF患者进行早期预测。 方法 选取北部战区总医院2019年1月—2023年10月244例行胰十二指肠切除术的患者,经过严格的纳入排除标准筛选后最终纳入179例患者,根据是否发生CR-POPF分为非CR-POPF组(n=120)和CR-POPF组(n=59)。采用单因素和多因素Logistic回归分析确定CR-POPF相关的独立危险因素,并构建列线图。采用受试者工作特征曲线评价预测效果,校准曲线评价模型校准度,用临床决策曲线和临床影响曲线分析验证模型的临床应用价值。计数资料组间比较采用χ2检验或Fisher精确概率法;计量资料符合正态分布的2组间比较采用成组t检验,偏态分布的2组间比较采用Mann-Whitney U检验。 结果 179例患者中59例发生CR-POPF,发生率为33.0%。经过多因素Logistic分析确定术后CR-POPF的独立危险因素:较大的ALT/AST(OR=2.221,P=0.004)、主胰管直径≤3 mm(OR=0.276,P=0.022)、较大的腹膜胰颈前距离(OR=1.034,P=0.027)、较小的细胞外体积分数(OR=0.001,P=0.005)。根据上述4个独立危险因素构建预测胰十二指肠术后CR-POPF的列线图,该模型的受试者工作特征曲线下面积为0.837,敏感度为0.932,特异度为0.725。决策曲线和影响曲线的结果也显示该列线图具有良好的临床实用性。 结论 术前临床指标联合多期CT共同预测胰十二指肠切除术后CR-POPF的模型效能良好,可以在术前对胰瘘高危患者进行早期识别,进一步指导临床工作。 Abstract:Objective To investigate the risk factors for clinically relevant postoperative pancreatic fistula (CR-POPF) after pancreaticoduodenectomy (PD), and to establish a predictive model for early identification of CR-POPF. Methods A total of 244 patients who underwent PD in General Hospital of Northern Theater Command from January 2019 to October 2023 were collected, and based on strict inclusion and exclusion criteria, 179 patients were finally enrolled in this study. According to the presence or absence of CR-POPF, these patients were divided into non-CR-POPF group with 120 patients and CR-POPF group with 59 patients. Univariate and multivariate logistic regression analyses were used to determine the independent risk factors for CR-POPF, and a nomogram model was established based on such factors. The receiver operating characteristic (ROC) curve was used to assess the predictive performance of the model, the calibration curve was used to evaluate the calibration degree of the model, and the clinical decision curve and the clinical impact curve were used to analyze and validate the clinical application value of the model. The chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups; the independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of continuous data with skewed distribution between two groups. Results Among the 179 patients, 59 (33.0%) developed CR-POPF. The multivariate Logistic regression analysis showed that alanine aminotransferase/aspartate aminotransferase (odds ratio [OR]=2.221, P=0.004), main pancreatic duct diameter (OR=0.276, P=0.022), the distance between the peritoneum and the anterior pancreatic neck (OR=1.034, P=0.027), and extracellular volume fraction (OR=0.001, P=0.005) were independent risk factors for CR-POPF. Based on the above four independent risk factors, a nomogram was established to predict CR-POPF after PD, with an area under the ROC curve of 0.837, a sensitivity of 0.932, and a specificity of 0.725. The decision curve and the clinical impact curve also showed that the nomogram had good clinical practicability. Conclusion Preoperative clinical indicators combined with multi-phase CT have a good performance in predicting CR-POPF after PD, which can be used to early identify patients at high risk of pancreatic fistula before surgery and provide further guidance for clinical work. -
Key words:
- Pancreaticoduodenectomy /
- Computed Tomography /
- Risk Factors /
- Pancreatic Fistula /
- Nomograms
-
表 1 患者临床基线资料
Table 1. Clinical baseline data of patients
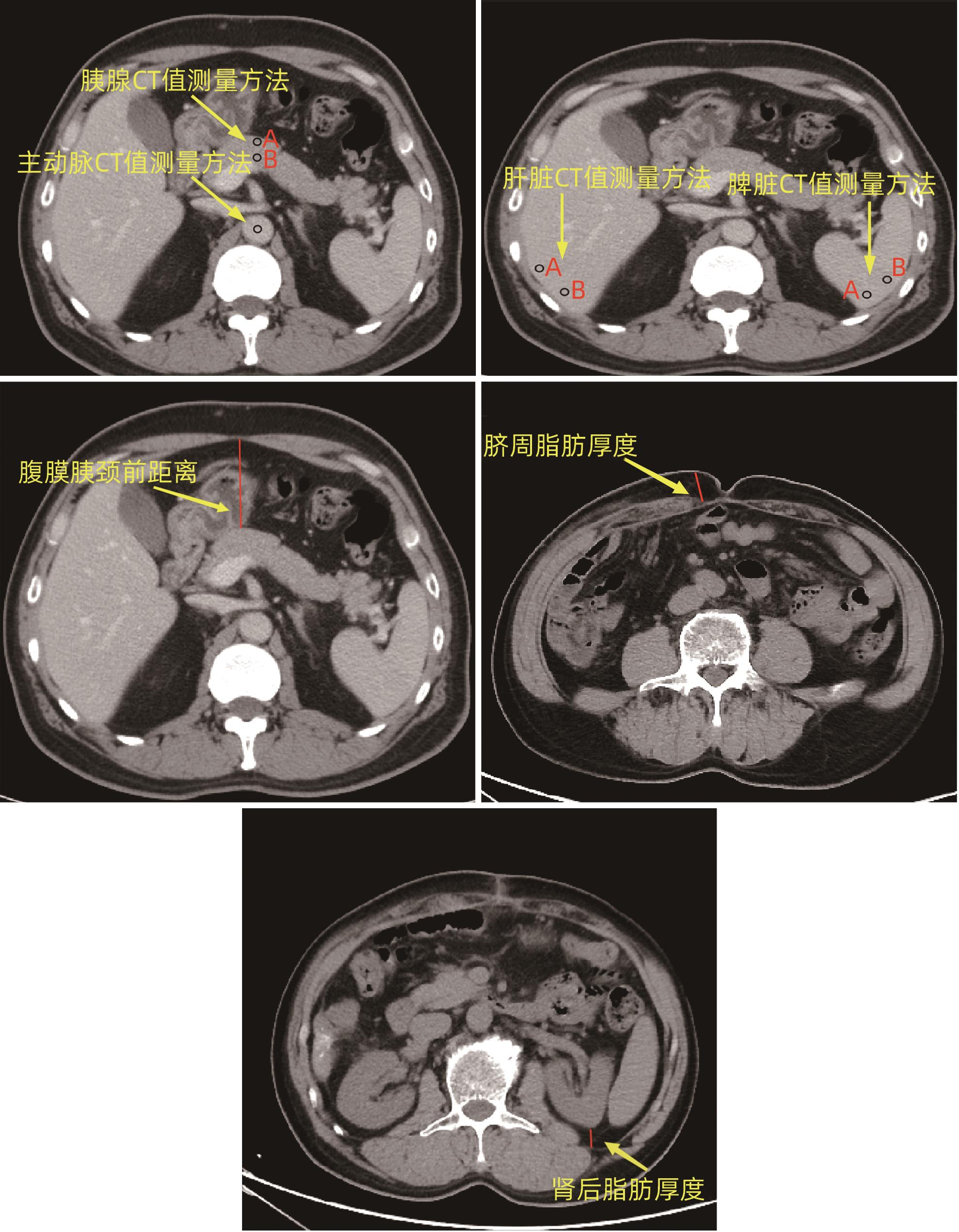
变量 数值 性别[例(%)] 女 63(35.2) 男 116(64.8) 年龄(岁) 62.0(56.0~69.0) 糖尿病[例(%)] 否 141(78.8) 是 38(21.2) 高血压[例(%)] 否 125(69.8) 是 54(30.2) BMI(kg/m2) 23.38±3.23 手术方式[例(%)] PD 129(72.1) LPD 50(27.9) 术前减黄[例(%)] 否 120(67.0) 是 59(33.0) 主胰管直径[例(%)] ≤3 mm 120(67.0) >3 mm 59(33.0) CT判断血管侵犯[例(%)] 否 162(90.5) 是 17(9.5) CR-POPF[例(%)] 否 120(67.0) 是 59(33.0) 术前血细胞比容 0.38(0.35~0.41) 术前血红蛋白(g/L) 129.00(118.00~138.00) 术前前白蛋白(mg/L) 170.17±55.70 术前白蛋白(g/L) 37.10(34.20~39.20) 术前总胆红素(μmol/L) 101.30(18.50~210.10) ALT(U/L) 126.19(41.81~237.50) AST(U/L) 82.82(30.60~151.45) ALT/AST 1.40(1.04~1.85) CA19-9(KU/L) 77.12(15.83~335.60) 胰腺平扫期CT值(Hu) 41.10(35.05~46.13) 胰腺动脉期CT值(Hu) 84.80(69.50~96.33) 胰腺静脉期CT值(Hu) 95.95(81.33~109.95) 胰腺平衡期CT值(Hu) 81.05±14.73 脾脏平扫期CT值(Hu) 52.65(48.83~55.30) 肝脏平扫期CT值(Hu) 56.20±6.61 主动脉平扫期CT值(Hu) 43.30(38.35~47.50) 主动脉平衡期CT值(Hu) 115.70(105.55~126.35) 胰/脾CT比值 0.77±0.15 胰/肝CT比值 0.72±0.15 肾后脂肪厚度(mm) 8.80(4.30~15.75) 脐周脂肪厚度(mm) 16.55(12.40~22.71) 腹膜胰颈前距离(mm) 50.43±1.19 ECV分数 0.33(0.27~0.39) 表 2 PD术后CR-POPF单因素分析
Table 2. Univariate analysis of the CR-POPF after PD
变量 非CR-POPF(n=120) CR-POPF(n=59) 统计值 P值 性别[例(%)] χ2=3.680 0.055 女 48(40.0) 15(25.4) 男 72(60.0) 44(74.6) 年龄[例(%)] χ2=0.574 0.449 ≤60岁 56(46.7) 24(40.7) >60岁 64(53.3) 35(59.3) 糖尿病[例(%)] χ2=1.826 0.177 否 98(81.7) 43(72.9) 是 22(18.3) 16(27.1) 高血压[例(%)] χ2=0.005 0.945 否 84(70.0) 41(69.5) 是 36(30.0) 18(30.5) BMI[例(%)] χ2=3.455 0.063 ≤25 kg/m2 93(77.5) 38(64.4) >25 kg/m2 27(22.5) 21(35.6) 手术方式[例(%)] χ2=1.556 0.272 开腹 90(75.0) 39(66.1) 腹腔镜 30(25.0) 20(33.9) 术前减黄[例(%)] χ2=0.276 0.599 否 82(68.3) 38(64.4) 是 38(31.7) 21(35.6) CT判断血管侵犯[例(%)] χ2=0.046 0.830 否 109(90.8) 53(89.8) 是 11(9.2) 6(10.2) 主胰管直径[例(%)] χ2=14.992 <0.001 ≤3 mm 69(57.5) 51(86.4) >3 mm 51(42.5) 8(13.6) 术前血细胞比容 0.37(0.35~0.41) 0.39(0.34~0.42) Z=-1.166 0.244 术前血红蛋白(g/L) 126.00(118.00~134.00) 132.00(116.00~142.00) Z=-1.862 0.063 术前前白蛋白(mg/L) 169.86±52.96 170.80±56.81 t=-0.109 0.913 术前白蛋白(g/L) 36.90(33.90~39.10) 37.80(34.60~40.30) Z=-1.047 0.295 术前总胆红素(μmol/L) 99.50(16.82~209.93) 112.50(34.50~221.40) Z=-0.655 0.512 ALT(U/L) 119.95(40.57~231.06) 143.62(44.98~229.75) Z=-0.331 0.740 AST(U/L) 85.44(31.86~169.99) 77.73(30.20~121.69) Z=-0.902 0.367 ALT/AST 1.27(1.00~1.68) 1.53(1.21~2.02) Z=-3.188 0.001 CA19-9(KU/L) 94.82(10.39~487.13) 53.90(25.47~257.90) Z=-0.124 0.901 胰腺平扫期CT值(Hu) 41.33(35.65~46.00) 39.05(34.70~46.15) Z=-0.813 0.416 胰腺动脉期CT值(Hu) 84.17(68.71~95.56) 88.00(76.25~92.00) Z=-1.837 0.066 胰腺静脉期CT值(Hu) 96.63(82.79~109.51) 95.70(81.10~110.30) Z=-0.175 0.861 胰腺平衡期CT值(Hu) 85.90(75.13~92.95) 71.50(67.70~79.40) Z=-4.833 <0.001 脾脏平扫期CT值(Hu) 52.58(48.8~55.21) 52.50(49.45~54.95) Z=-0.075 0.94 肝脏平扫期CT值(Hu) 56.01±6.76 56.59±6.33 t=-0.546 0.586 主动脉平扫期CT值(Hu) 42.56±7.06 42.15±6.48 t=0.369 0.713 主动脉平衡期CT值(Hu) 116.20(105.78~130.78) 116.30(107.40~125.80) Z=-0.074 0.941 胰/脾CT比值 0.80(0.67~0.89) 0.76(0.66~0.87) Z=-0.942 0.346 胰/肝CT比值 0.73±0.16 0.71±0.13 t=0.978 0.329 肾后脂肪厚度(mm) 8.24(3.80~14.51) 11.60(6.30~16.50) Z=-2.068 0.039 脐周脂肪厚度(mm) 15.95(12.10~21.71) 19.10(14.30~25.30) Z=-2.189 0.029 腹膜胰颈前距离(mm) 46.99±16.31 57.43±12.56 t=-4.718 <0.001 ECV分数 0.36(0.32~0.41) 0.29(0.24~0.31) Z=-6.045 <0.001 表 3 多因素Logistic分析
Table 3. Multivariate Logistic regression analysis
变量 β值 OR(95%CI) P值 主胰管直径(≤3 mm vs >3 mm) -1.117 0.276(0.105~0.725) 0.022 ALT/AST 0.798 2.221(1.281~3.851) 0.004 胰腺平衡期CT值 -0.008 0.992(0.956~1.030) 0.691 肾后脂肪厚度 -0.01 0.990(0.937~1.047) 0.732 脐周脂肪厚度 0.002 1.002(0.956~1.050) 0.937 腹膜胰颈前距离 0.034 1.034(1.004~1.066) 0.027 ECV分数 -9.126 0.001(0.000~0.067) 0.005 表 4 相关指标预测效能
Table 4. Prediction efficiency of relevant indicators
变量 AUC(95%CI) 敏感度 特异度 PPV NPV Cut-off ALT/AST 0.647(0.560~0.734) 0.729 0.542 0.439 0.802 1.322 主胰管直径 0.645(0.582~0.707) 0.864 0.425 0.425 0.864 腹膜胰颈前距离 0.686(0.607~0.765) 0.966 0.342 0.419 0.953 38.7 ECV分数 0.778(0.709~0.847) 0.847 0.717 0.595 0.905 0.325 Nomogram 0.837(0.766~0.898) 0.932 0.725 0.625 0.956 264 -
[1] AUGUSTINUS S, MACKAY TM, ANDERSSON B, et al. Ideal outcome after pancreatoduodenectomy: A transatlantic evaluation of a harmonized composite outcome measure[J]. Ann Surg, 2023, 278( 5): 740- 747. DOI: 10.1097/SLA.0000000000006037. [2] WANG Z, LYU X, YU JA, et al. Pathogenesis of delayed gastric emptying after pancreaticoduodenectomy and related risk factors[J]. J Clin Hepatol, 2023, 39( 2): 474- 480. DOI: 10.3969/j.issn.1001-5256.2023.02.036.王哲, 吕行, 于家傲, 等. 胰十二指肠切除术后胃排空延迟的发病机制及危险因素[J]. 临床肝胆病杂志, 2023, 39( 2): 474- 480. DOI: 10.3969/j.issn.1001-5256.2023.02.036. [3] GAO HQ, LI BY, MA YS, et al. Risk factors analysis and treatment of postpancreaticoduodenectomy hemorrhage[J]. Chin J Dig Surg, 2022, 21( 4): 492- 499. DOI: 10.3760/cma.j.cn115610-20220228-00111.高红桥, 李宝毅, 马永蔌, 等. 胰十二指肠切除术后出血的危险因素分析及治疗策略[J]. 中华消化外科杂志, 2022, 21( 4): 492- 499. DOI: 10.3760/cma.j.cn115610-20220228-00111. [4] MARCHEGIANI G. The 2016 update of the International Study Group(ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After[J]. HPB, 2019, 21: S748. DOI: 10.1016/j.hpb.2019.10.1473. [5] AMBROSETTI MC, AMBROSETTI A, PERRI G, et al. Quantitative edge analysis of pancreatic margins in patients with head pancreatic tumors: Correlations between pancreatic margins and the onset of postoperative pancreatic fistula[J]. Eur Radiol, 2024, 34( 3): 1515- 1523. DOI: 10.1007/s00330-023-10200-6. [6] LIU L, XU ZH, WANG WQ, et al. Prevention and management of pancreatic fistula after pancreatoduodenectomy with precise and comprehensive opinion[J]. Chin J Dig Surg, 2023, 22( 5): 657- 662. DOI: 10.3760/cma.j.cn115610-20230401-00143.刘亮, 徐志航, 王文权, 等. 精准联合综合策略防治胰十二指肠切除术后胰瘘[J]. 中华消化外科杂志, 2023, 22( 5): 657- 662. DOI: 10.3760/cma.j.cn115610-20230401-00143. [7] POTTER KC, SUTTON TL, O’GRADY J, et al. Risk factors for postoperative pancreatic fistula in the Era of pasireotide[J]. Am J Surg, 2022, 224( 2): 733- 736. DOI: 10.1016/j.amjsurg.2022.02.050. [8] RAMACCIATO G, MERCANTINI P, PETRUCCIANI N, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy: A collective review[J]. Am Surg, 2011, 77( 3): 257- 269. [9] KAMARAJAH SK, BUNDRED JR, LIN A, et al. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy[J]. ANZ J Surg, 2021, 91( 5): 810- 821. DOI: 10.1111/ans.16408. [10] ZHANG B, YUAN QH, LI S, et al. Risk factors of clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy: A systematic review and meta-analysis[J]. Medicine, 2022, 101( 26): e29757. DOI: 10.1097/MD.0000000000029757. [11] LI Y, SHI YB, TU JH, et al. Risk factors and prophylaxis for pancreatic fistula after pancreaticoduodenectomy[J/OL]. Chin J Hepat Surg(Electronic Edition), 2023, 12( 3): 352- 355. DOI: 10.3877/cma.j.issn.2095-3232.2023.03.021.李扬, 史亚波, 涂建华, 等. 胰十二指肠切除术后胰瘘发生的危险因素及预防[J/OL]. 中华肝脏外科手术学电子杂志, 2023, 12( 3): 352- 355. DOI: 10.3877/cma.j.issn.2095-3232.2023.03.021. [12] RAZA SS, NUTU A, POWELL-BRETT S, et al. Early postoperative risk stratification in patients with pancreatic fistula after pancreaticoduodenectomy[J]. Surgery, 2023, 173( 2): 492- 500. DOI: 10.1016/j.surg.2022.09.008. [13] GUO CX, SHEN YN, ZHANG Q, et al. Prediction of postoperative pancreatic fistula using a nomogram based on the updated definition[J]. Ann Surg Treat Res, 2020, 98( 2): 72- 81. DOI: 10.4174/astr.2020.98.2.72. [14] SHEN J, GUO F, SUN Y, et al. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: A retrospective study[J]. BMC Cancer, 2021, 21( 1): 550. DOI: 10.1186/s12885-021-08201-z. [15] CHOI M, LEE JH, ROH YH, et al. Multidimensional nomogram to predict postoperative pancreatic fistula after minimally invasive pancreaticoduodenectomy[J]. Ann Surg Oncol, 2023, 30( 8): 5083- 5090. DOI: 10.1245/s10434-023-13360-3. [16] YOU Y, HAN IW, CHOI DW, et al. Nomogram for predicting postoperative pancreatic fistula[J]. HPB(Oxford), 2019, 21( 11): 1436- 1445. DOI: 10.1016/j.hpb.2019.03.351. [17] ZHOU Z, WANG R, WANG H, et al. Myocardial extracellular volume fraction quantification in an animal model of the doxorubicin-induced myocardial fibrosis: A synthetic hematocrit method using 3T cardiac magnetic resonance[J]. Quant Imaging Med Surg, 2021, 11( 2): 510- 520. DOI: 10.21037/qims-20-501. [18] CAVALCANTE JL, KOIKE H. The rise of myocardial extracellular volume fraction in computed tomography for identification of cardiac amyloidosis[J]. JACC Cardiovasc Imaging, 2022, 15( 12): 2095- 2097. DOI: 10.1016/j.jcmg.2022.09.010. [19] MESROPYAN N, KUPCZYK P, ISAAK A, et al. Synthetic extracellular volume fraction without hematocrit sampling for hepatic applications[J]. Abdom Radiol, 2021, 46( 10): 4637- 4646. DOI: 10.1007/s00261-021-03140-6. [20] TAKAHASHI M, TAKAOKA H, YASHIMA S, et al. Extracellular volume fraction by computed tomography predicts prognosis after transcatheter aortic valve replacement[J]. Circ J, 2024, 88( 4): 492- 500. DOI: 10.1253/circj.CJ-23-0288. [21] OZAKI K, OHTANI T, ISHIDA S, et al. Extracellular volume fraction obtained by dual-energy CT depicting the etiological differences of liver fibrosis[J]. Abdom Radiol, 2023, 48( 6): 1975- 1986. DOI: 10.1007/s00261-023-03873-6. [22] ZHU L, SUN ZY, DAI MH, et al. Tomoelastography and pancreatic extracellular volume fraction derived from MRI for predicting clinically relevant postoperative pancreatic fistula[J]. J Magn Reson Imaging, 2024, 59( 3): 1074- 1082. DOI: 10.1002/jmri.28788. [23] SOFUE K, UESHIMA E, MASUDA A, et al. Estimation of pancreatic fibrosis and prediction of postoperative pancreatic fistula using extracellular volume fraction in Multiphasic contrast-enhanced CT[J]. Eur Radiol, 2022, 32( 3): 1770- 1780. DOI: 10.1007/s00330-021-08255-4. [24] Study Group of Pancreatic Surgery in Chinese Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of Chinese Research Hospital Association, Editorial Board of Chinese Journal of Surgery. A consensus statement on the diagnosis, treatment, and prevention of common complications after pancreatic surgery(2017)[J]. Chin J Surg, 2017, 55( 5): 328- 334. DOI: 10.3760/cma.j.issn.0529-5815.2017.05.003.中华医学会外科学分会胰腺外科学组, 中国研究型医院学会胰腺病专业委员会, 中华外科杂志编辑部. 胰腺术后外科常见并发症诊治及预防的专家共识(2017)[J]. 中华外科杂志, 2017, 55( 5): 328- 334. DOI: 10.3760/cma.j.issn.0529-5815.2017.05.003. [25] HANAKI T, UEJIMA C, AMISAKI M, et al. The attenuation value of preoperative computed tomography as a novel predictor for pancreatic fistula after pancreaticoduodenectomy[J]. Surg Today, 2018, 48( 6): 598- 608. DOI: 10.1007/s00595-018-1626-y. [26] KIM SY, KIM H, CHO JY, et al. Quantitative assessment of pancreatic fat by using unenhanced CT: Pathologic correlation and clinical implications[J]. Radiology, 2014, 271( 1): 104- 112. DOI: 10.1148/radiol.13122883. [27] ZHAO ZR, ZHOU LC, HAN L, et al. The visceral pancreatic neck anterior distance may be an effective parameter to predict post-pancreaticoduodenectomy clinically relevant postoperative pancreatic fistula[J]. Heliyon, 2023, 9( 2): e13660. DOI: 10.1016/j.heliyon.2023.e13660. [28] TANAKA K, YAMADA S, SONOHARA F, et al. Pancreatic fat and body composition measurements by computed tomography are associated with pancreatic fistula after pancreatectomy[J]. Ann Surg Oncol, 2021, 28( 1): 530- 538. DOI: 10.1245/s10434-020-08581-9. [29] CALLERY MP, PRATT WB, KENT TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy[J]. J Am Coll Surg, 2013, 216( 1): 1- 14. DOI: 10.1016/j.jamcollsurg.2012.09.002. [30] GU ZT, DU YX, WANG P, et al. Development and validation of a novel nomogram to predict postoperative pancreatic fistula after pancreatoduodenectomy using lasso-logistic regression: An international multi-institutional observational study[J]. Int J Surg, 2023, 109( 12): 4027- 4040. DOI: 10.1097/JS9.0000000000000695. [31] WANG CY, OU HY, CHEN MF, et al. Enigmatic ectopic fat: Prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population[J]. J Am Heart Assoc, 2014, 3( 1): e000297. DOI: 10.1161/JAHA.113.000297. [32] PECORELLI N, PALUMBO D, GUARNERI G, et al. Preoperative CT image analysis to improve risk stratification for clinically relevant pancreatic fistula after distal pancreatectomy[J]. Br J Surg, 2023, 110( 8): 891- 895. DOI: 10.1093/bjs/znac348. [33] LEE SE, JANG JY, LIM CS, et al. Measurement of pancreatic fat by magnetic resonance imaging: Predicting the occurrence of pancreatic fistula after pancreatoduodenectomy[J]. Ann Surg, 2010, 251( 5): 932- 936. DOI: 10.1097/SLA.0b013e3181d65483. [34] SALTIEL AR, OLEFSKY JM. Inflammatory mechanisms linking obesity and metabolic disease[J]. J Clin Invest, 2017, 127( 1): 1- 4. DOI: 10.1172/JCI92035. [35] WANG ZY, LIU RH, LI FS, et al. Analysis of relative factors of pancreatic leakage after laparoscopic pancreaticoduodenectomy[J]. Chin J Minim Invasive Surg, 2019, 19( 2): 106- 110. DOI: 10.3969/j.issn.1009-6604.2019.02.003.王振勇, 刘汝海, 李凤山, 等. 腹腔镜胰十二指肠切除术后胰漏的相关因素分析[J]. 中国微创外科杂志, 2019, 19( 2): 106- 110. DOI: 10.3969/j.issn.1009-6604.2019.02.003. [36] SERT OZ, BERKESOGLU M, CANBAZ H, et al. The factors of pancreatic fistula development in patients who underwent classical pancreaticoduodenectomy[J]. Ann Ital Chir, 2021, 92: 35- 40. [37] ZHOU LC, TAN Z, TANG YP, et al. Value of pancreatic anatomic structure under standard pancreatic neck transection in predicting pancreatic fistula after pancreaticoduodenectomy[J]. J Clin Hepatol, 2022, 38( 12): 2807- 2813. DOI: 10.3969/j.issn.1001-5256.2022.12.022.周黎晨, 谭震, 唐娅萍, 等. 标准胰颈横断下胰腺断面结构参数对胰十二指肠切除术后胰瘘的预测价值[J]. 临床肝胆病杂志, 2022, 38( 12): 2807- 2813. DOI: 10.3969/j.issn.1001-5256.2022.12.022. [38] SCHUH F, MIHALJEVIC AL, PROBST P, et al. A simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: A classification of the international study group of pancreatic surgery[J]. Ann Surg, 2023, 277( 3): e597- e608. DOI: 10.1097/SLA.0000000000004855. [39] HAN D, LIN A, KURONUMA K, et al. Cardiac computed tomography for quantification of myocardial extracellular volume fraction: A systematic review and meta-analysis[J]. JACC Cardiovasc Imaging, 2023, 16( 10): 1306- 1317. DOI: 10.1016/j.jcmg.2023.03.021. [40] OZAKI K, OHTANI T, ISHIDA T, et al. Liver fibrosis estimated using extracellular volume fraction obtained from dual-energy CT as a risk factor for hepatocellular carcinoma after sustained virologic response: A preliminary case-control study[J]. Eur J Radiol, 2023, 168: 111112. DOI: 10.1016/j.ejrad.2023.111112. [41] GUO WX, LV BL, YANG T, et al. Role of dynamic contrast-enhanced magnetic resonance imaging parameters and extracellular volume fraction as predictors of lung cancer subtypes and lymph node status in non-small-cell lung cancer patients[J]. J Cancer, 2023, 14( 16): 3108- 3116. DOI: 10.7150/jca.88367. -



 PDF下载 ( 2208 KB)
PDF下载 ( 2208 KB)

 下载:
下载: