细胞焦亡: 连接肠道菌群与肝脏疾病的新桥梁
DOI: 10.12449/JCH240930
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:赵奕杰负责拟定写作思路,撰写文章;谢露负责调整文章架构和修改;张亚亭负责文献收集整理;刘光伟指导文章撰写和修改。
-
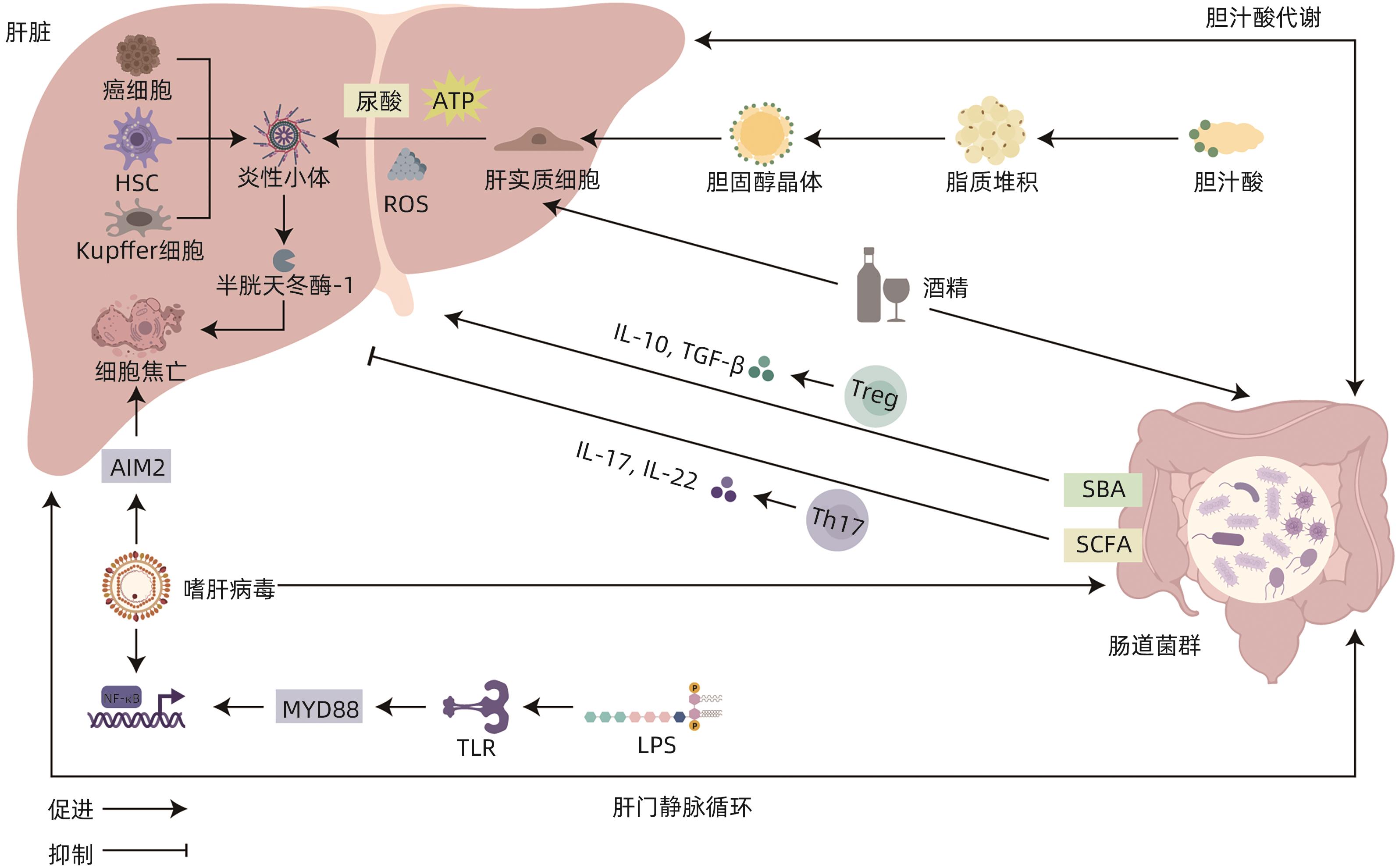
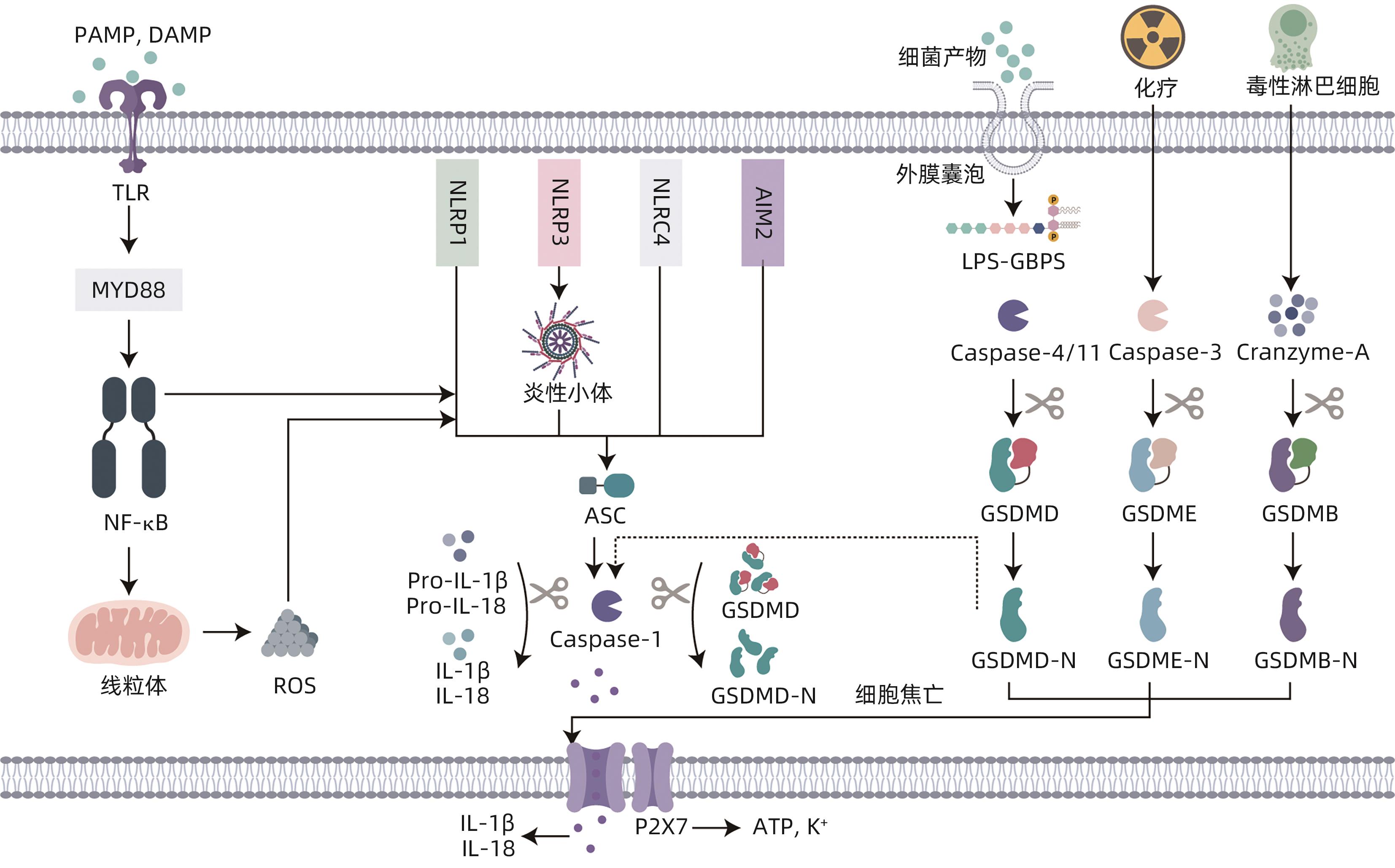
摘要: 自肠-肝轴的概念被提出以来,已有大量研究着眼于探索肠道菌群和肝病之间的联系,但以细胞焦亡为枢纽,探究肠-肝串扰的内在机制的观点仍处于萌芽阶段。本文主要通过叙述了肠道菌群失调通过影响黏膜屏障的完整性和胆汁酸的代谢,诱导细胞焦亡,进而影响肝脏相关疾病的发生和进展的过程,并总结出肠道菌群失调通过诱导NLRP3/AIM2/Caspase-1依赖型、Caspase-4/11/GSDMD依赖型和Caspase-3/GSDME依赖型细胞焦亡以影响肝脏相关疾病的观点。希望通过建立细胞焦亡与肠-肝免疫串扰之间的联系,为未来肝病的诊治提供新的思路和靶点。Abstract: Since the proposal of the concept of the gut-liver axis, a large number of studies have focused on exploring the connection between gut microbiota and liver disease; however, the idea of using pyroptosis as a hub to explore the intrinsic mechanism of gut-liver crosstalk is still in its infancy. This article mainly describes the process by which gut microbiota dysbiosis affects the integrity of mucosal barrier and bile acid metabolism, induces pyroptosis, and thereby affects the development and progression of liver diseases, and it also concludes that gut microbiota dysbiosis affects liver diseases by inducing NLRP3/AIM2/Caspase-1-dependent, Caspase-4/11/GSDMD-dependent, and Caspase-3/GSDME-dependent pyroptosis. In summary, this study aims to provide new ideas and targets for the future diagnosis and treatment of liver diseases by establishing the connection between pyroptosis and intestinal-liver immune crosstalk.
-
Key words:
- Liver Diseases /
- Gastrointestinal Microbiome /
- Pyroptosis /
- Bile Acids and Salts
-
[1] de VOS WM, TILG H, van HUL M, et al. Gut microbiome and health: Mechanistic insights[J]. Gut, 2022, 71( 5): 1020- 1032. DOI: 10.1136/gutjnl-2021-326789. [2] PABST O, HORNEF MW, SCHAAP FG, et al. Gut-liver axis: Barriers and functional circuits[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 7): 447- 461. DOI: 10.1038/s41575-023-00771-6. [3] KNORR J, WREE A, FELDSTEIN AE. Pyroptosis in steatohepatitis and liver diseases[J]. J Mol Biol, 2022, 434( 4): 167271. DOI: 10.1016/j.jmb.2021.167271. [4] GIUFFRÈ M, CAMPIGOTTO M, CAMPISCIANO G, et al. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature[J]. Am J Physiol Gastrointest Liver Physiol, 2020, 318( 5): G889- G906. DOI: 10.1152/ajpgi.00161.2019. [5] KNORR J, WREE A, FELDSTEIN AE. Pyroptosis in steatohepatitis and liver diseases[J] Mol Biol, 2022, 434( 4): 167271. DOI: 10.1016/j.jmb.2021.167271. [6] CHOPYK DM, GRAKOUI A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159( 3): 849- 863. DOI: 10.1053/j.gastro.2020.04.077. [7] ECKBURG PB, BIK EM, BERNSTEIN CN, et al. Diversity of the human intestinal microbial flora[J]. Science, 2005, 308( 5728): 1635- 1638. DOI: 10.1126/science.1110591. [8] MOWAT AM, AGACE WW. Regional specialization within the intestinal immune system[J]. Nat Rev Immunol, 2014, 14( 10): 667- 685. DOI: 10.1038/nri3738. [9] BEL S, PENDSE M, WANG YH, et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine[J]. Science, 2017, 357( 6355): 1047- 1052. DOI: 10.1126/science.aal4677. [10] MÖRBE UM, JØRGENSEN PB, FENTON TM, et al. Human gut-associated lymphoid tissues(GALT); diversity, structure, and function[J]. Mucosal Immunol, 2021, 14( 4): 793- 802. DOI: 10.1038/s41385-021-00389-4. [11] YANG WJ, CONG YZ. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases[J]. Cell Mol Immunol, 2021, 18( 4): 866- 877. DOI: 10.1038/s41423-021-00661-4. [12] ANDERSON MJ, DEN HARTIGH AB, FINK SL. Molecular mechanisms of pyroptosis[J]. Methods Mol Biol, 2023, 2641: 1- 16. DOI: 10.1007/978-1-0716-3040-2_1. [13] SANTOS JC, DICK MS, LAGRANGE B, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation[J]. EMBO J, 2018, 37( 6): e98089. DOI: 10.15252/embj.201798089. [14] WANG JF, DONG R, ZHENG S. Roles of the inflammasome in the gut-liver axis(Review)[J]. Mol Med Rep, 2019, 19( 1): 3- 14. DOI: 10.3892/mmr.2018.9679. [15] FERNANDES-ALNEMRI T, WU J, YU JW, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation[J]. Cell Death Differ, 2007, 14( 9): 1590- 1604. DOI: 10.1038/sj.cdd.4402194. [16] SHAO F. Gasdermins: Making pores for pyroptosis[J]. Nat Rev Immunol, 2021, 21( 10): 620- 621. DOI: 10.1038/s41577-021-00602-2. [17] CHEN KW, DEMARCO B, BROZ P. Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation[J]. Eur J Immunol, 2020, 50( 2): 170- 177. DOI: 10.1002/eji.201948254. [18] HUANG XL, FENG Y, XIONG GQ, et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis[J]. Cell Biosci, 2019, 9: 31. DOI: 10.1186/s13578-019-0292-0. [19] WANG YP, GAO WQ, SHI XY, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547( 7661): 99- 103. DOI: 10.1038/nature22393. [20] ZHOU ZW, HE HB, WANG K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells[J]. Science, 2020, 368( 6494): eaaz7548. DOI: 10.1126/science.aaz7548. [21] BARNETT KC, LI SR, LIANG KX, et al. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases[J]. Cell, 2023, 186( 11): 2288- 2312. DOI: 10.1016/j.cell.2023.04.025. [22] ZHANG YW, YANG WL, LI WG, et al. NLRP3 inflammasome: Checkpoint connecting innate and adaptive immunity in autoimmune diseases[J]. Front Immunol, 2021, 12: 732933. DOI: 10.3389/fimmu.2021.732933. [23] MARTENS EC, NEUMANN M, DESAI MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier[J]. Nat Rev Microbiol, 2018, 16( 8): 457- 470. DOI: 10.1038/s41579-018-0036-x. [24] XUE SG, XUE Y, DOU DB, et al. Kui Jie Tong ameliorates ulcerative colitis by regulating gut microbiota and NLRP3/caspase-1 classical pyroptosis signaling pathway[J]. Dis Markers, 2022, 2022: 2782112. DOI: 10.1155/2022/2782112. [25] SZABO G, CSAK T. Inflammasomes in liver diseases[J]. J Hepatol, 2012, 57( 3): 642- 654. DOI: 10.1016/j.jhep.2012.03.035. [26] HAO HP, CAO LJ, JIANG CT, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis[J]. Cell Metab, 2017, 25( 4): 856- 867.e5. DOI: 10.1016/j.cmet.2017.03.007. [27] PERINO A, SCHOONJANS K. Metabolic messengers: Bile acids[J]. Nat Metab, 2022, 4( 4): 416- 423. DOI: 10.1038/s42255-022-00559-z. [28] HAN CY, RHO HS, KIM A, et al. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury[J]. Cell Rep, 2018, 24( 11): 2985- 2999. DOI: 10.1016/j.celrep.2018.07.068. [29] CHEN Y, LE TH, DU QM, et al. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling[J]. Int Immunopharmacol, 2019, 71: 144- 154. DOI: 10.1016/j.intimp.2019.01.021. [30] HANG SY, PAIK D, YAO LN, et al. Bile acid metabolites control TH17 and Treg cell differentiation[J]. Nature, 2019, 576( 7785): 143- 148. DOI: 10.1038/s41586-019-1785-z. [31] HUANG FJ, ZHENG XJ, MA XH, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism[J]. Nat Commun, 2019, 10( 1): 4971. DOI: 10.1038/s41467-019-12896-x. [32] MANNS MP, LOHSE AW, VERGANI D. Autoimmune hepatitis-update 2015[J]. J Hepatol, 2015, 62( 1): S100- S111. DOI: 10.1016/j.jhep.2015.03.005. [33] LIU QY, HE W, TANG RQ, et al. Intestinal homeostasis in autoimmune liver diseases[J]. Chin Med J, 2022, 135( 14): 1642- 1652. DOI: 10.1097/CM9.0000000000002291. [34] LI LP, KANG YB. The gut microbiome and autoimmune hepatitis: Implications for early diagnostic biomarkers and novel therapies[J]. Mol Nutr Food Res, 2023, 67( 24): e2300043. DOI: 10.1002/mnfr.202300043. [35] CHEN JN, WEI YF, HE JQ, et al. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota[J]. Sci Rep, 2014, 4: 7259. DOI: 10.1038/srep07259. [36] KANG YB, KUANG XY, YAN H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway[J]. mSystems, 2023, 8( 2): e0112722. DOI: 10.1128/msystems.01127-22. [37] WANG H, WANG GD, LIANG YJ, et al. Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity[J]. Free Radic Biol Med, 2019, 143: 223- 231. DOI: 10.1016/j.freeradbiomed.2019.08.014. [38] SEITZ HK, BATALLER R, CORTEZ-PINTO H, et al. Alcoholic liver disease[J]. Nat Rev Dis Primers, 2018, 4( 1): 16. DOI: 10.1038/s41572-018-0014-7. [39] CHO YE, SONG BJ. Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress[J]. Redox Biol, 2018, 18: 266- 278. DOI: 10.1016/j.redox.2018.07.012. [40] PETRASEK J, IRACHETA-VELLVE A, SAHA B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease[J]. J Leukoc Biol, 2015, 98( 2): 249- 256. DOI: 10.1189/jlb.3AB1214-590R. [41] WU S, WEN F, ZHONG XB, et al. Astragaloside IV ameliorate acute alcohol-induced liver injury in mice via modulating gut microbiota and regulating NLRP3/caspase-1 signaling pathway[J]. Ann Med, 2023, 55( 1): 2216942. DOI: 10.1080/07853890.2023.2216942. [42] KHANOVA E, WU R, WANG W, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients[J]. Hepatology, 2018, 67( 5): 1737- 1753. DOI: 10.1002/hep.29645. [43] CALIGIURI A, GENTILINI A, MARRA F. Molecular pathogenesis of NASH[J]. Int J Mol Sci, 2016, 17( 9): 1575. DOI: 10.3390/ijms17091575. [44] MUSSO G, CASSADER M, GAMBINO R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies[J]. Nat Rev Drug Discov, 2016, 15( 4): 249- 274. DOI: 10.1038/nrd.2015.3. [45] BASHIARDES S, SHAPIRO H, ROZIN S, et al. Non-alcoholic fatty liver and the gut microbiota[J]. Mol Metab, 2016, 5( 9): 782- 794. DOI: 10.1016/j.molmet.2016.06.003. [46] TIAN GG, WANG W, XIA ER, et al. Dendrobium officinale alleviates high-fat diet-induced nonalcoholic steatohepatitis by modulating gut microbiota[J]. Front Cell Infect Microbiol, 2023, 13: 1078447. DOI: 10.3389/fcimb.2023.1078447. [47] CSAK T, GANZ M, PESPISA J, et al. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells[J]. Hepatology, 2011, 54( 1): 133- 144. DOI: 10.1002/hep.24341. [48] ZHU YW, ZHAO H, LU J, et al. Caspase-11-mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis[J]. Cell Mol Gastroenterol Hepatol, 2021, 12( 2): 653- 664. DOI: 10.1016/j.jcmgh.2021.04.009. [49] YANG JJ, WANG DW, LI YC, et al. Metabolomics in viral hepatitis: Advances and review[J]. Front Cell Infect Microbiol, 2023, 13: 1189417. DOI: 10.3389/fcimb.2023.1189417. [50] SEHGAL R, BEDI O, TREHANPATI N. Role of microbiota in pathogenesis and management of viral hepatitis[J]. Front Cell Infect Microbiol, 2020, 10: 341. DOI: 10.3389/fcimb.2020.00341. [51] LU YX, HE CZ, WANG YX, et al. Effect of entecavir on the intestinal microflora in patients with chronic hepatitis B: A controlled cross-sectional and longitudinal real-world study[J]. Infect Dis Ther, 2021, 10( 1): 241- 252. DOI: 10.1007/s40121-020-00355-w. [52] INOUE T, NAKAYAMA J, MORIYA K, et al. Gut dysbiosis associated with hepatitis C virus infection[J]. Clin Infect Dis, 2018, 67( 6): 869- 877. DOI: 10.1093/cid/ciy205. [53] NEGASH AA, RAMOS HJ, CROCHET N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease[J]. PLoS Pathog, 2013, 9( 4): e1003330. DOI: 10.1371/journal.ppat.1003330. [54] YU X, LAN PX, HOU XB, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production[J]. J Hepatol, 2017, 66( 4): 693- 702. DOI: 10.1016/j.jhep.2016.12.018. [55] XIE WH, DING J, XIE XX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation[J]. Inflamm Res, 2020, 69( 7): 683- 696. DOI: 10.1007/s00011-020-01351-z. [56] PAN XF, XU HX, ZHENG CL, et al. Human hepatocytes express absent in melanoma 2 and respond to hepatitis B virus with interleukin-18 expression[J]. Virus Genes, 2016, 52( 4): 445- 452. DOI: 10.1007/s11262-016-1327-9. [57] HAN Y, CHEN Z, HOU R, et al. Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients[J]. Virol J, 2015, 12: 129. DOI: 10.1186/s12985-015-0360-y. [58] HO DWH, LO RCL, CHAN LK, et al. Molecular pathogenesis of hepatocellular carcinoma[J]. Liver Cancer, 2016, 5( 4): 290- 302. DOI: 10.1159/000449340. [59] LUO WY, GUO SQ, ZHOU Y, et al. Hepatocellular carcinoma: How the gut microbiota contributes to pathogenesis, diagnosis, and therapy[J]. Front Microbiol, 2022, 13: 873160. DOI: 10.3389/fmicb.2022.873160. [60] BI CC, XIAO GQ, LIU CY, et al. Molecular immune mechanism of intestinal microbiota and their metabolites in the occurrence and development of liver cancer[J]. Front Cell Dev Biol, 2021, 9: 702414. DOI: 10.3389/fcell.2021.702414. [61] ZHAO HJ, ZHANG YM, ZHANG YT, et al. The role of NLRP3 inflammasome in hepatocellular carcinoma[J]. Front Pharmacol, 2023, 14: 1150325. DOI: 10.3389/fphar.2023.1150325. [62] LIU BY, ZHOU ZW, JIN Y, et al. Hepatic stellate cell activation and senescence induced by intrahepatic microbiota disturbances drive progression of liver cirrhosis toward hepatocellular carcinoma[J]. J Immunother Cancer, 2022, 10( 1): e003069. DOI: 10.1136/jitc-2021-003069. [63] CHEN WB, DING M, JI LY, et al. Bile acids promote the development of HCC by activating inflammasome[J]. Hepatol Commun, 2023, 7( 9): e0217. DOI: 10.1097/HC9.0000000000000217. [64] WEI Q, GUO PB, MU K, et al. Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome[J]. Lab Invest, 2015, 95( 7): 804- 816. DOI: 10.1038/labinvest.2015.63. [65] ZHANG Y, YANG H, SUN MF, et al. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis[J]. Pharmacol Rep, 2020, 72( 5): 1370- 1382. DOI: 10.1007/s43440-020-00064-8. [66] BHAT AA, THAPA R, AFZAL O, et al. The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review[J]. Int J Biol Macromol, 2023, 242( Pt 2): 124832. DOI: 10.1016/j.ijbiomac.2023.124832. [67] SHANGGUAN FG, ZHOU HF, MA NF, et al. A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis[J]. Front Cell Dev Biol, 2021, 9: 697832. DOI: 10.3389/fcell.2021.697832. -



 PDF下载 ( 1609 KB)
PDF下载 ( 1609 KB)

 下载:
下载: