老年HBV相关慢加急性肝衰竭预后的危险因素及风险预测列线图模型构建
DOI: 10.12449/JCH241009
Risk factors for the prognosis of elderly patients with hepatitis B virus-related acute-on-chronic liver failure and construction of a nomogram model for risk prediction
-
摘要:
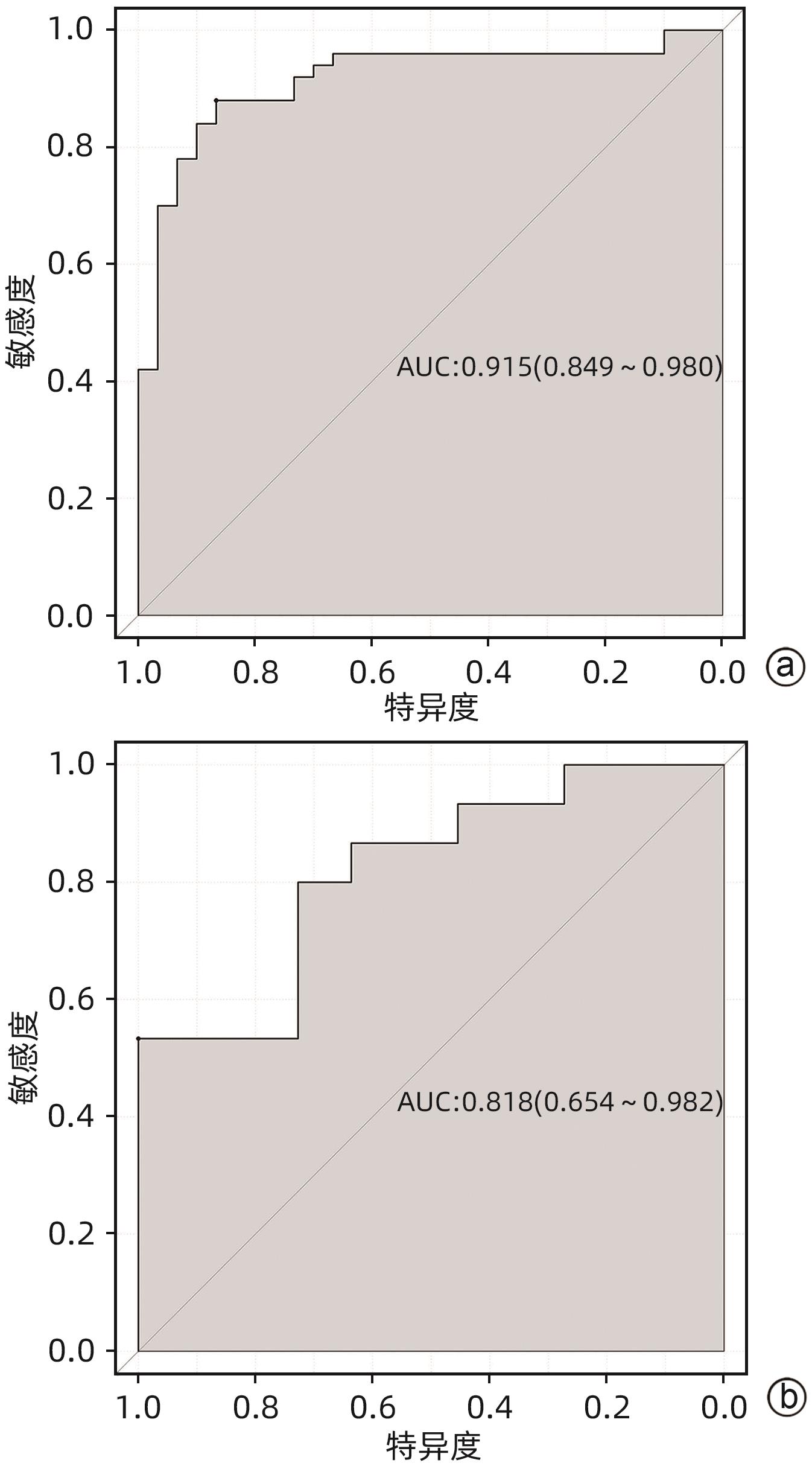
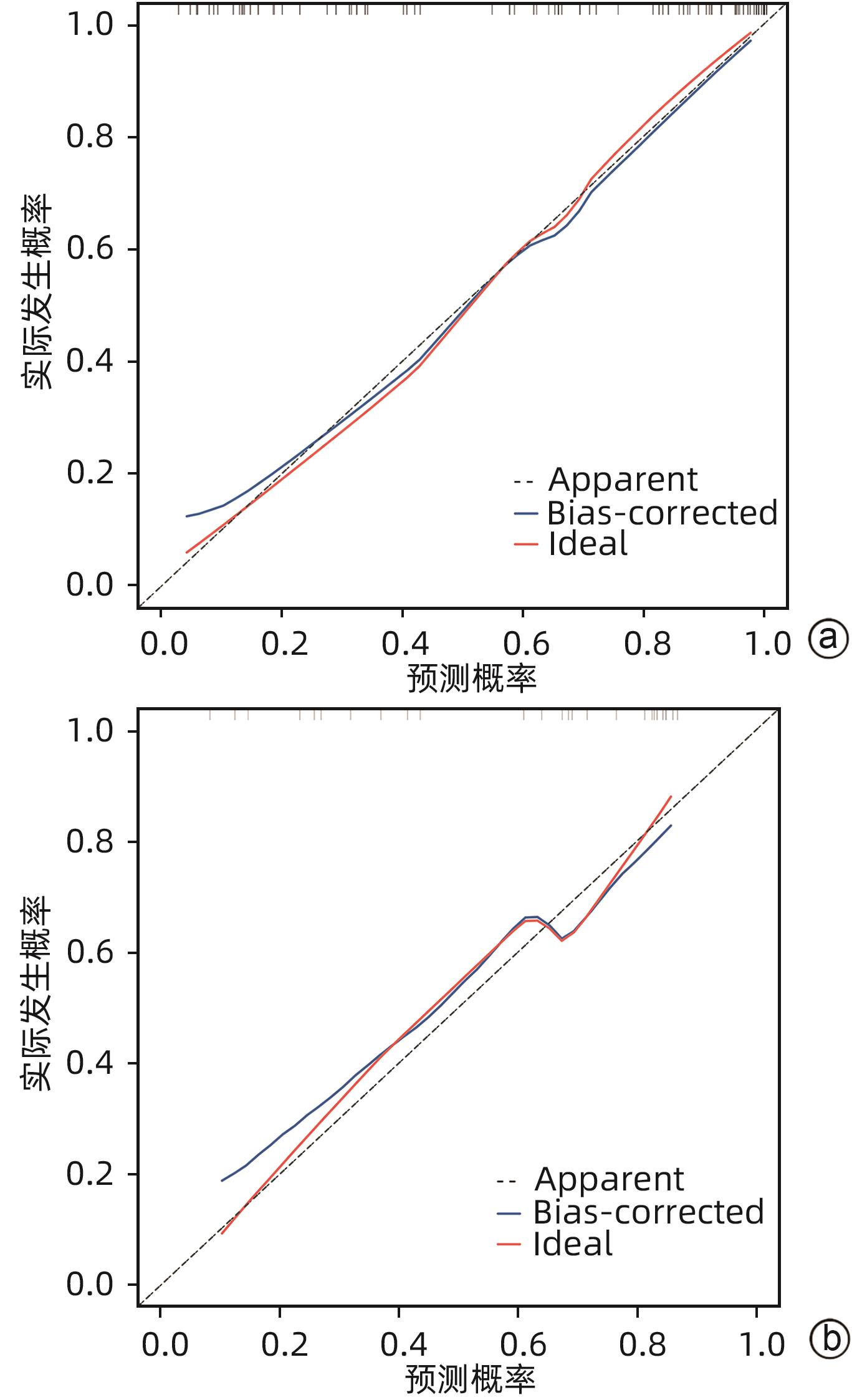
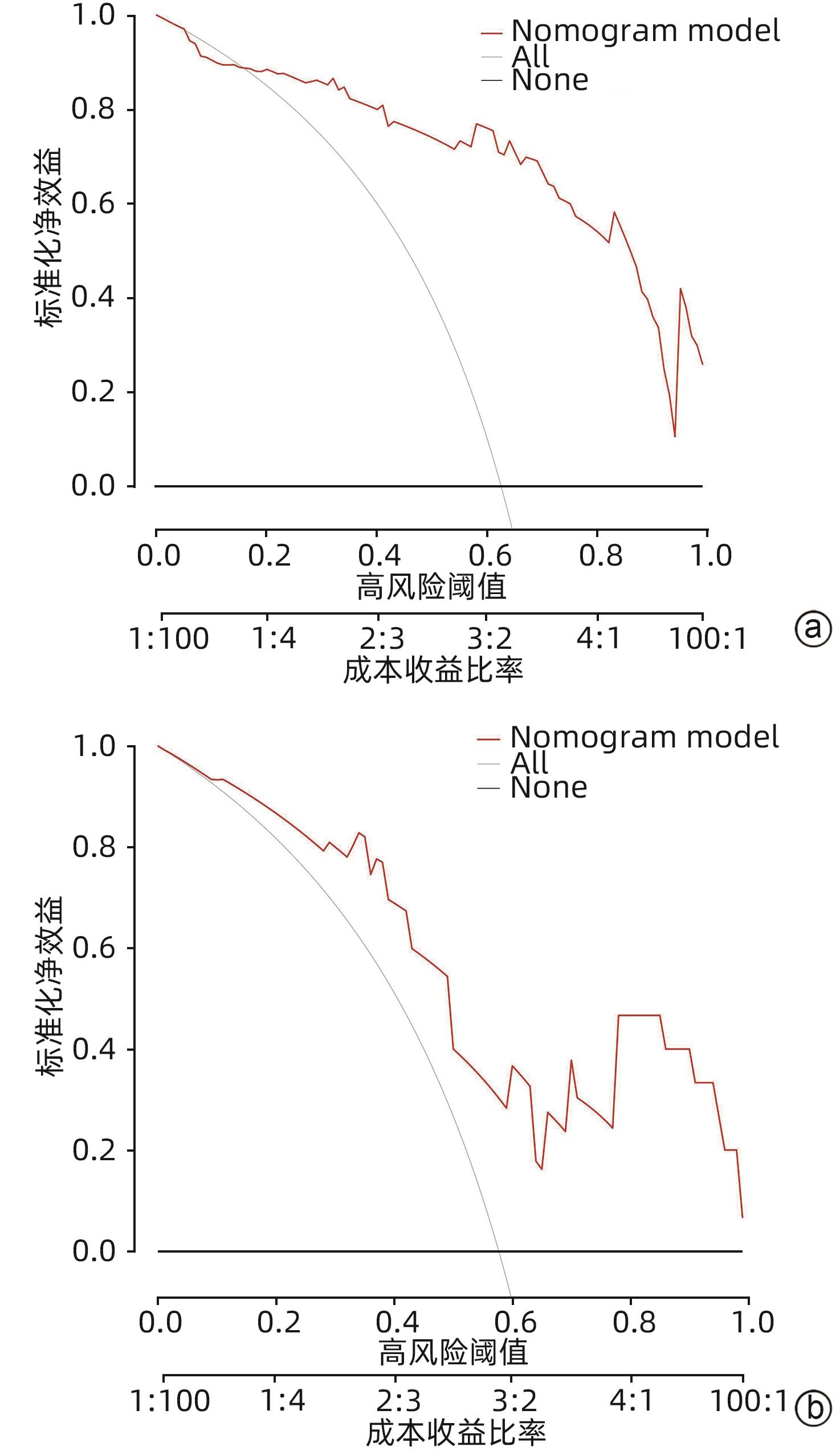
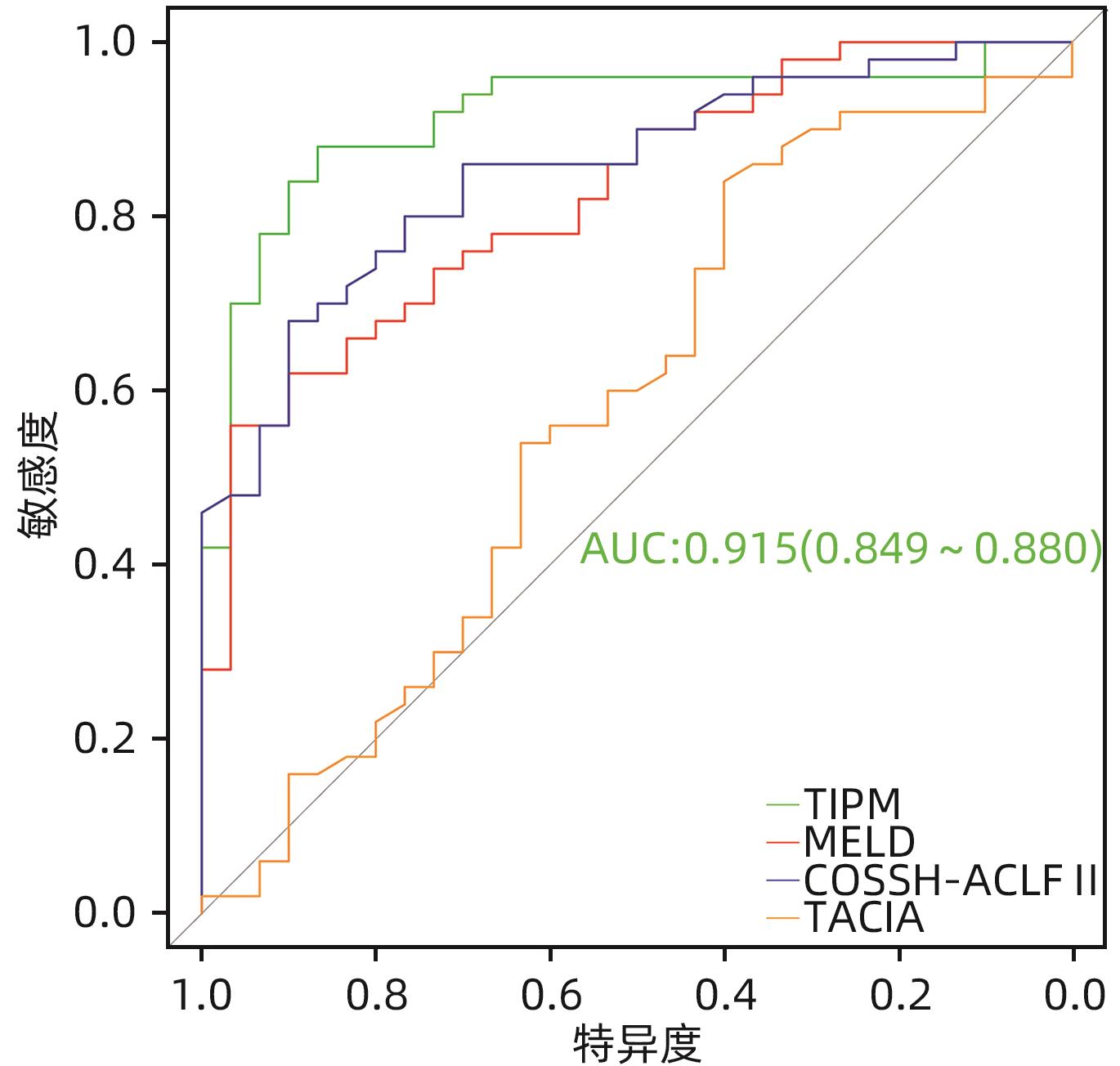
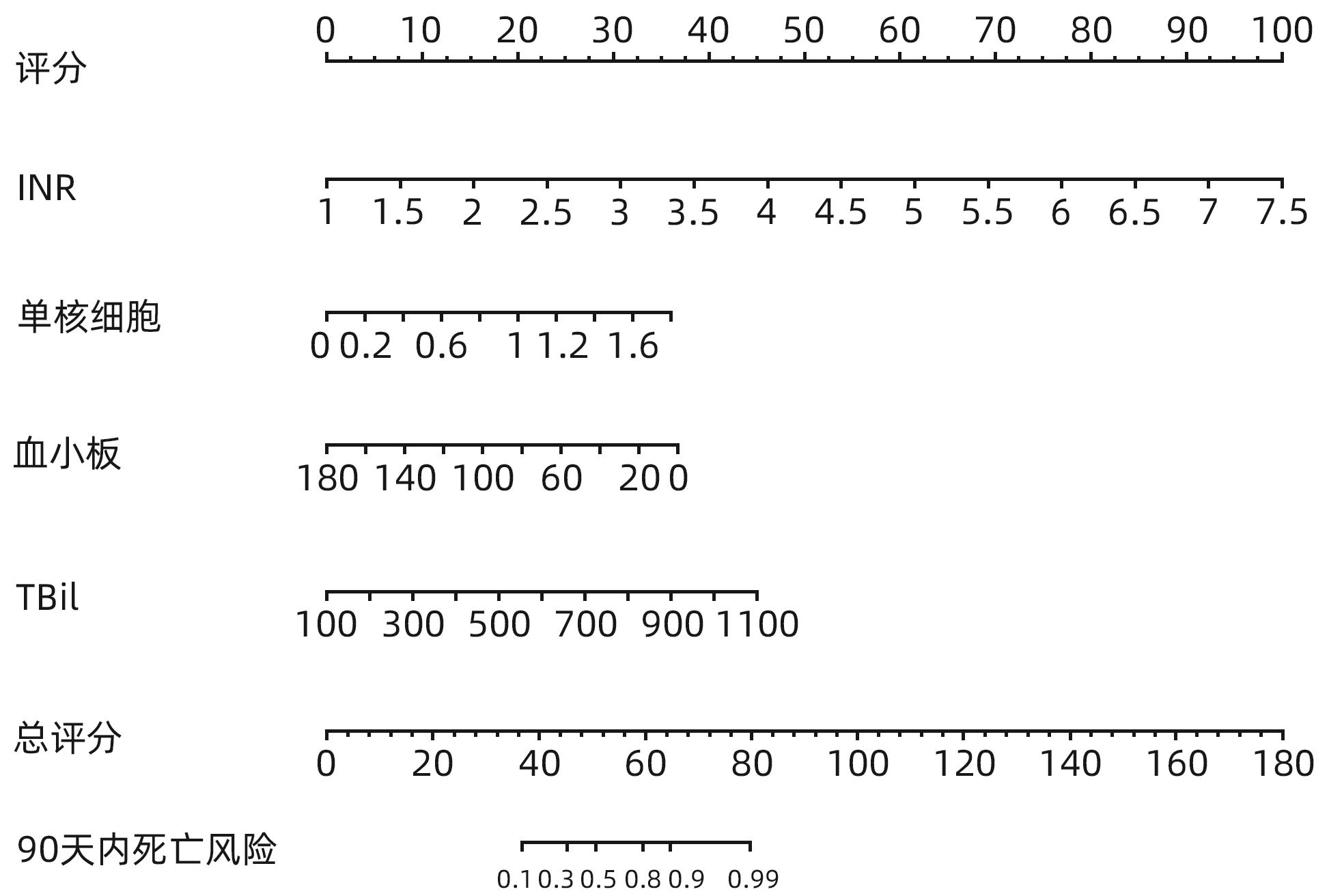
目的 探讨老年HBV相关慢加急性肝衰竭(HBV-ACLF)患者的临床特点,以及影响患者近期预后的危险因素。 方法 选取2015年1月—2023年1月在西部战区总医院收治的417例HBV-ACLF患者进行回顾性研究。收集患者一般情况、血常规、生化指标、肝硬化及失代偿事件情况(腹水及分级,肝性脑病及分级)等临床资料。随访患者90天生存情况。根据年龄将患者分为老年组(≥60岁,n=106)和非老年组(<60岁,n=311),老年组中根据90天生存情况分为生存组(n=41)和死亡或移植组(n=65)。定量资料两组间比较采用成组t检验或Mann-Whitney U检验,定性资料两组间比较采用χ2检验。采用二元Logistic回归分析确定老年HBV-ACLF患者90天死亡风险的独立影响因素,构建老年HBV-ACLF患者死亡风险预测列线图模型。使用受试者工作特征曲线(ROC曲线)分别评价训练集与验证集中模型对于HBV-ACLF患者转归预测价值。绘制训练集与验证集中所构建模型的校准曲线与决策曲线,判断模型拟合程度与预测收益。 结果 老年患者的90天病死率显著高于非老年患者(P<0.05),其中老年组女性发生率、肝硬化基础发生率、肝性脑病发生率及分级、腹水发生率、肝纤维化指标水平(APRI、FIB-4)显著高于非老年组(P值均<0.05);而总胆固醇、高密度脂蛋白、白蛋白、甲胎蛋白、淋巴细胞等方面显著低于非老年组(P值均<0.05)。在老年HBV-ACLF患者中,生存组与死亡或移植组在总胆固醇、TBil、国际化标准比值(INR)、甲胎蛋白、血小板、肌酐、血清钠、单核细胞数量、肝性脑病发生率及分级等方面存在显著性差异(P值均<0.05)。此外,多因素Logistic回归分析提示INR(OR=11.351,95%CI:1.942~66.362)、单核细胞(OR=23.636,95%CI:1.388~402.529)、TBil(OR=1.007,95%CI:1.001~1.013)、血小板(OR=0.968,95%CI:0.945~0.993)为HBV-ACLF老年患者90天预后的独立影响因素(P值均<0.05),由此构建的列线图模型具有较高预测价值(ROC曲线下面积为0.915,敏感度为88.0%,特异度为86.7%),且在验证集中同样具有较高效能与拟合度,决策曲线提示获益较好,该模型与常用预测模型(MELD评分、COSSH-ACLFⅡ评分等)相比,仍具有较高预测效能。 结论 老年HBV-ACLF患者可能因肝脏合成、储备功能、再生能力下降、免疫功能紊乱等原因而表现为短期高病死率。INR、单核细胞、TBil、血小板在预测老年HBV-ACLF患者死亡风险方面具有较高价值,由此构建的列线图模型具有较高预测效能。 Abstract:Objective To investigate the clinical features of elderly patients with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) and the risk factors affecting the short-term prognosis of patients. Methods A retrospective analysis was performed for 417 patients with HBV-ACLF who were admitted to The General Hospital of Western Theater Command from January 2015 to January 2023, and related clinical data were collected, including general status, routine blood test results, biochemical parameters, and conditions of liver cirrhosis and decompensated events (ascites, hepatic encephalopathy, and their severities). The patients were followed up to observe 90-day survival. According to the age, the patients were divided into elderly group (with 106 patients aged ≥60 years) and non-elderly group (with 311 patients aged<60 years), and according to the 90-day survival, the elderly group were further divided into survival group with 41 patients and death/transplantation group with 65 patients. The independent-samples t test or the Mann-Whitney U test was used for comparison of quantitative data between two groups, and the chi-square test was used for comparison of qualitative data between two groups. The binary logistic regression analysis was used to determine the independent influencing factors for the risk of death within 90 days in elderly patients with HBV-ACLF, and a nomogram model was constructed for predicting the risk of death. The receiver operating characteristic (ROC) curve was used to investigate the value of the model in predicting the prognosis of HBV-ACLF patients in both the training set and the validation set. Calibration curve and decision curve were plotted for the models constructed in the training set and the validation set, and the model was assessed in terms of the degree of fitness and predicting benefits. Results The elderly patients had a significantly higher 90-day mortality rate than the non-elderly patients (P<0.05), and compared with the non-elderly group, the elderly group had significantly higher incidence rate in female individuals, basic incidence rate of liver cirrhosis, incidence rate and grade of hepatic encephalopathy, incidence rate of ascites, and liver fibrosis markers (aspartate aminotransferase-to-platelet ratio index and fibrosis-4) (all P<0.05), as well as significantly lower total cholesterol, high-density lipoprotein, albumin, alpha-fetoprotein, and lymphocytes (all P<0.05). As for the elderly patients with HBV-ACLF, there were significant differences between the survival group and the death/transplantation group in total cholesterol, total bilirubin, international normalized ratio (INR), alpha-fetoprotein, platelet, creatinine, serum sodium, monocytes, and the incidence rate and grade of hepatic encephalopathy (all P<0.05). In addition, the multivariate logistic regression analysis showed that INR (odds ratio [OR]=11.351, 95% confidence interval [CI]: 1.942 — 66.362, P<0.05), monocyte count (OR=23.636, 95%CI: 1.388 — 402.529, P<0.05), total bilirubin (OR=1.007, 95%CI: 1.001 — 1.013, P<0.05), and platelet count (OR=0.968, 95%CI: 0.945 — 0.993, P<0.05) were independent influencing factors for the 90-day prognosis of elderly patients with HBV-ACLF, and the nomogram model constructed based on these factors had a relatively high predictive value, with an area under the ROC curve of 0.915, a sensitivity of 88.0%, and a specificity of 86.7%. The nomogram model showed relatively high efficiency and degree of fitness in the verification set, and the decision curve suggested that the model had good benefits, with a higher prediction efficiency compared with the commonly used prediction models such as MELD score and COSSH-ACLF Ⅱ score. Conclusion Elderly HBV-ACLF patients may have a high short-term mortality rate due to the reductions in liver synthesis, reserve function, and regenerative ability and immune dysfunction. INR, monocyte count, total bilirubin, and platelet count have a relatively high value in predicting the risk of death in elderly HBV-ACLF patients, and the nomogram model constructed based on these factors has a relatively high prediction efficiency. -
Key words:
- Acute-On-Chronic Liver Failure /
- Aged /
- Nomograms /
- Prognosis
-
表 1 老年组和非老年组临床特征比较
Table 1. Comparison of clinical features between elderly group and non-elderly group
指标 非老年组(n=311) 老年组(n=106) 统计值 P值 男性[例(%)] 270(81.817) 79(74.528) χ2=8.747 0.003 90天病死率[例(%)] 100(32.154) 65(61.320) χ2=28.124 <0.001 白细胞(×109/L) 6.175(4.592~8.198) 5.720(4.580~7.805) Z=-0.965 0.335 血小板(×109/L) 98.766±75.747 83.248±39.374 t=2.017 0.044 中性粒细胞(×109/L) 4.365(3.035~6.420) 4.370(3.137~5.798) Z=0.154 0.878 淋巴细胞(×109/L) 0.980(0.740~1.412) 0.705(0.487~1.070) Z=-4.885 <0.001 单核细胞(×109/L) 0.545(0.390~0.780) 0.540(0.360~0.780) Z=-0.699 0.485 TBil(μmol/L) 321.370±152.156 353.437±155.964 t=-1.862 0.063 ALT(U/L) 445.250(157.600~1 085.325) 430.900(142.675~784.125) Z=-0.751 0.453 AST(U/L) 341.850(145.675~801.100) 388.500(182.050~953.900) Z=1.412 0.158 白蛋白(g/L) 31.600±5.204 30.262±3.705 t=2.442 0.015 总胆固醇(mmol/L) 2.030(1.497~2.683) 1.785(1.337~2.423) Z=-2.583 0.010 HDL-C(mmol/L) 0.330(0.210~0.552) 0.270(0.137~0.465) Z=-2.268 0.023 AFP(ng/mL) 76.295(19.105~217.312) 22.120(11.992~67.895) Z=-4.485 <0.001 INR 1.780(1.570~2.170) 1.880(1.607~2.273) Z=1.065 0.287 肌酐(μmol/L) 74.900(64.900~89.000) 78.00(63.000~104.000) Z=1.350 0.177 血钠(mmol/L) 135.500(132.515~138.055) 134.950(131.675~138.025) Z=-1.590 0.122 APRI 9.925(4.572~21.755) 12.435(5.362~27.358) Z=2.502 0.012 FIB-4 8.885(5.055~16.795) 18.835(9.200~30.403) Z=7.308 <0.001 MELD评分 22.195(19.467~25.513) 23.345(19.117~27.300) Z=1.896 0.058 COSSH-ACLFⅡ评分 6.810(6.217~7.353) 7.725(7.192~8.588) Z=8.859 <0.001 肝硬化基础[例(%)] 185(59.486) 87(82.075) χ2=17.787 <0.001 上消化道出血[例(%)] 28(9.000) 7(6.604) χ2=0.592 0.442 腹水[例(%)] 263(84.566) 99(93.396) χ2=5.384 0.020 肝性脑病[例(%)] 62(19.936) 42(39.623) χ2=16.367 <0.001 肝性脑病分级[例(%)] χ2=16.898 <0.001 无 249(80.064) 64(60.377) 1~2级 39(12.540) 29(27.359) 3~4级 23(7.396) 13(12.264) 表 2 老年HBV-ACLF生存组和死亡或移植组临床特征比较
Table 2. Comparison of clinical features between survival group and death/transplantation group in HBV-ACLF
指标 生存组(n=41) 死亡或移植组(n=65) 统计值 P值 男性[例(%)] 30(73.170) 49(75.385) χ2=0.065 0.799 白细胞(×109/L) 5.320(4.230~6.860) 5.730(4.995~8.755) Z=1.606 0.108 血小板(×109/L) 97.220±42.658 74.435±34.674 t=3.011 0.003 中性粒细胞(×109/L) 4.250(2.995~5.480) 4.440(3.570~6.695) Z=1.284 0.199 单核细胞(×109/L) 0.440(0.340~0.585) 0.600(0.385~0.845) Z=2.492 0.013 TBil(μmol/L) 286.556±114.221 395.623±164.562 t=-3.714 <0.001 ALT(U/L) 399.700(148.950~738.950) 424.700(136.900~807.000) Z=-0.204 0.838 AST(U/L) 393.300(174.750~1 034.650) 383.300(194.250~969.800) Z=-0.139 0.889 GGT(U/L) 119.800(76.600~174.150) 87.900(63.900~151.900) Z=-1.495 0.135 总胆汁酸(μmol/L) 210.800(135.900~251.200) 220.800(163.700~277.900) Z=1.064 0.293 白蛋白(g/L) 30.406±3.890 30.172±3.612 t=0.315 0.754 前白蛋白(mg/L) 45.056±20.667 37.363±24.712 t=1.660 0.100 总胆固醇(mmol/L) 2.223±0.817 1.740±0.742 t=3.136 0.002 HDL-C(mmol/L) 0.372±0.205 0.308±0.207 t=1.571 0.119 AFP(ng/mL) 55.830(12.670~115.040) 18.100(10.810~48.650) Z=-2.163 0.031 INR 1.678±0.285 2.351±0.943 t=-4.439 <0.001 血红蛋白(g/L) 121.000(110.500~131.000) 121.000(104.000~136.000) Z=-0.133 0.894 肌酐(μmol/L) 79.998±29.715 101.932±62.634 t=-2.096 0.039 续表
表 3 训练集与验证集老年HBV-ACLF患者临床特征比较
Table 3. Comparison of clinical features of elderly HBV-ACLF patients in training and verification
指标 训练集(n=80) 验证集(n=26) 统计值 P值 白细胞(×109/L) 5.700(4.770~7.768) 5.795(3.735~8.428) Z=-0.389 0.697 血小板(×109/L) 83.741±36.086 81.731±48.922 t=0.225 0.822 中性粒细胞(×109/L) 4.395(3.510~5.730) 4.240(2.915~6.275) Z=-0.272 0.786 单核细胞(×109/L) 0.550(0.390~0.810) 0.510(0.278~0.680) Z=-1.091 0.275 TBil(μmol/L) 352.883±155.048 355.143±161.844 t=-0.064 0.949 ALT(U/L) 404.600(136.425~942.525) 492.100(169.975~673.475) Z=0.073 0.941 AST(U/L) 382.150(190.700~1 003.820) 473.200(209.325~917.600) Z=0.206 0.837 GGT(U/L) 94.750(64.675~170.250) 104.550(67.325~168.525) Z=0.268 0.789 总胆汁酸(μmol/L) 222.250(167.625~274.375) 200.655(146.030~280.350) Z=-0.720 0.472 白蛋白(g/L) 30.240±3.899 30.333±3.102 t=-0.111 0.912 前白蛋白(mg/L) 39.067±23.811 44.251±22.216 t=-0.980 0.329 总胆固醇(mmol/L) 1.930±0.820 1.934±0.769 t=-0.045 0.964 HDL-C(mmol/L) 0.320(0.153~0.478) 0.265(0.198~0.375) Z=-0.672 0.502 AFP(ng/mL) 28.700(11.998~68.845) 17.295(8.303~65.988) Z=-0.977 0.329 INR 2.147±0.927 1.918±0.330 t=1.229 0.222 血红蛋白(g/L) 121.500(110.000~135.700) 120.500(103.500~131.500) Z=-0.551 0.582 肌酐(μmol/L) 79.000(64.225~102.500) 78.500(61.825~108.050) Z=0.099 0.921 血清钠(mmol/L) 134.614±5.044 133.488±4.582 t=1.011 0.315 血清钾(mmol/L) 3.780(3.508~4.128) 3.885(3.453~4.378) Z=0.712 0.460 血清磷(mmol/L) 0.280(0.200~0.520) 0.515(0.250~0.723) Z=1.884 0.060 APRI 13.575(5.675~27.343) 14.325(6.045~38.193) Z=0.378 0.705 FIB-4 18.360(9.603~31.123) 24.130(11.355~42.863) Z=1.138 0.255 MELD评分 24.625±7.116 24.215±5.960 t=0.265 0.792 COSSH-ACLFⅡ评分 7.927±1.073 8.102±1.093 t=-0.722 0.472 肝性脑病[例(%)] 30(37.500) 12(46.154) χ2=0.614 0.433 腹水[例(%)] 75(93.750) 24(92.308) χ2=0.066 0.797 上消化道出血[例(%)] 6(7.500) 1(3.846) χ2=0.425 0.515 肝性脑病分级[例(%)] χ2=2.124 0.346 无 51(63.750) 13(50.000) 1~2级 21(26.250) 8(30.769) 3~4级 8(10.000) 5(19.231) 表 4 老年HBV-ACLF患者90天预后Logistic回归分析
Table 4. Logistic regression analysis of 90-day prognosis in elderly HBV-ACLF patients
指标 单因素Logistic分析 多因素Logistic分析 β值 OR(95%CI) P值 β值 OR(95%CI) P值 白细胞(×109/L) 0.079 1.082(0.951~1.232) 0.232 血小板(×109/L) -0.021 0.979(0.965~0.994) 0.006 -0.032 0.968(0.945~0.993) 0.011 中性粒细胞(×109/L) 0.081 1.084(0.942~1.248) 0.258 淋巴细胞(×109/L) 0.066 1.068(0.483~2.363) 0.871 单核细胞(×109/L) 1.672 5.324(1.037~27.340) 0.045 3.163 23.636(1.388~402.529) 0.029 TBil(μmol/L) 0.008 1.008(1.003~1.013) 0.001 0.007 1.007(1.001~1.013) 0.020 ALT(U/L) 0.001 1.000(0.999~1.001) 0.809 AST(U/L) 0.001 1.000(0.999~1.001) 0.647 GGT(U/L) -0.003 0.997(0.993~1.001) 0.104 总胆汁酸(μmol/L) 0.004 1.004(0.998~1.009) 0.182 白蛋白(g/L) 0.005 1.005(0.894~1.130) 0.931 前白蛋白(mg/L) -0.015 0.985(0.966~1.005) 0.140 总胆固醇(mmol/L) -1.34 0.262(0.119~0.577) 0.001 HDL-C(mmol/L) -3.454 0.032(0.003~0.383) 0.007 AFP(ng/mL) -0.002 0.998(0.996~1.001) 0.201 INR 3.179 24.029(4.055~142.377) <0.001 2.429 11.351(1.942~66.362) 0.007 血红蛋白(g/L) 0.008 1.008(0.984~1.032) 0.515 肌酐(μmol/L) 0.013 1.013(0.998~1.029) 0.079 血清钠(mmol/L) -0.194 0.824(0.723~0.938) 0.003 血钾(mmol/L) 0.714 2.042(0.846~4.928) 0.112 血磷(mmol/L) 0.853 2.346(0.488~11.268) 0.287 肝性脑病[例(%)] 1.952 7.042(2.142~23.145) 0.001 指标 生存组(n=41) 死亡或移植组(n=65) 统计值 P值 血清钠(mmol/L) 136.588±3.510 132.919±5.197 t=3.981 <0.001 血钾(mmol/L) 3.720(3.440~4.060) 3.890(3.550~4.215) Z=1.048 0.295 血磷(mmol/L) 0.310(0.215~0.560) 0.300(0.195~0.700) Z=0.136 0.872 APRI 10.205(4.855~27.389) 16.765(6.905~31.566) Z=1.268 0.205 FIB-4 16.917(8.513~29.942) 21.098(11.827~34.941) Z=1.703 0.089 MELD评分 20.734±4.164 26.915±7.113 t=-5.041 <0.001 COSSH-ACLFⅡ评分 7.337±0.560 8.370±1.132 t=-5.431 <0.001 腹水[例(%)] 36(87.805) 63(96.923) χ2=2.072 0.150 上消化道出血[例(%)] 2(4.878) 5(7.692) χ2=0.028 0.868 肝性脑病[例(%)] 6(14.634) 36(55.385) χ2=17.452 <0.001 肝性脑病分级[例(%)] χ2=19.072 <0.001 无 35(85.366) 29(44.615) 1~2级 6(14.634) 23(35.385) 3~4级 0(0.000) 13(20.000) 表 5 列线图与其他模型对老年HBV-ACLF患者预后的预测价值比较
Table 5. Comparison of the predictive value of TIPM and other models in elderly HBV-ACLF patients
评分模型 AUC 截断值 敏感度 特异度 95%CI P值 列线图 0.915 0.597 0.880 0.867 0.849~0.980 <0.001 MELD评分 0.825 25.015 0.560 0.967 0.735~0.914 <0.001 COSSH-ACLFⅡ评分 0.853 7.725 0.680 0.900 0.771~0.935 <0.001 TACIA评分 0.580 5.535 0.840 0.400 0.443~0.717 <0.001 -
[1] LI X, ZHANG L, PU CM, et al. Liver transplantation in acute-on-chronic liver failure: Timing of transplantation and selection of patient population[J]. Front Med(Lausanne), 2022, 9: 1030336. DOI: 10.3389/fmed.2022.1030336. [2] Hepatology Branch of Chinese Medical Association, Infectious Diseases Branch of Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Infect Dis, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050. [3] LIU L, HAN T, CAI JJ, et al. Clinical characteristics and progression risk factors for hepatitis B-related acute-on-chronic liver failure in elderly patients[J]. Chin J Geriatr, 2022, 41( 1): 51- 56. DOI: 10.3760/cma.j.issn.0254-9026.2022.01.011.刘磊, 韩涛, 蔡均均, 等. 老年乙肝相关慢加急性肝衰竭患者临床特点及进展危险因素分析[J]. 中华老年医学杂志, 2022, 41( 1): 51- 56. DOI: 10.3760/cma.j.issn.0254-9026.2022.01.011. [4] WANG X, ZHAO M, ZHANG C, et al. Establishment and clinical application of the nomogram related to risk or prognosis of hepatocellular carcinoma: A review[J]. J Hepatocell Carcinoma, 2023, 10: 1389- 1398. DOI: 10.2147/jhc.s417123. [5] ZHANG LJ, TANG L, CHEN SY, et al. A nomogram for predicting the 4-year risk of chronic kidney disease among Chinese elderly adults[J]. Int Urol Nephrol, 2023, 55( 6): 1609- 1617. DOI: 10.1007/s11255-023-03470-y. [6] CAI ZM, LIN HM, LI ZX, et al. A prediction nomogram for postoperative gastroparesis syndrome in right colon cancer: A retrospective study[J]. Langenbecks Arch Surg, 2023, 408( 1): 148. DOI: 10.1007/s00423-023-02885-6. [7] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [8] ZHAO RH, SHI Y, ZHAO H, et al. Acute-on-chronic liver failure in chronic hepatitis B: An update[J]. Expert Rev Gastroenterol Hepatol, 2018, 12( 4): 341- 350. DOI: 10.1080/17474124.2018.1426459. [9] KAKISAKA K, KATAOKA K, ONODERA M, et al. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure[J]. Hepatol Res, 2015, 45( 10): E12- E20. DOI: 10.1111/hepr.12448. [10] WANG XP, SHEN CF, YANG JJ, et al. Alpha-fetoprotein as a predictive marker for patients with hepatitis B-related acute-on-chronic liver failure[J]. Can J Gastroenterol Hepatol, 2018, 2018: 1232785. DOI: 10.1155/2018/1232785. [11] QIN S, TANG SH, WANG XH, et al. Value of serum alpha-fetoprotein for the prognostic evaluation of hepatitis B virus-related acute-on-chronic liver failure treated with artificial liver[J]. Chin J Hepatol, 2020, 28( 1): 69- 72. DOI: 10.3760/cma.j.issn.1007-3418.2020.01.016.秦森, 汤善宏, 王显红, 等. 血清甲胎蛋白在人工肝治疗乙型肝炎相关慢加急性肝衰竭预后评估中的价值[J]. 中华肝脏病杂志, 2020, 28( 1): 69- 72. DOI: 10.3760/cma.j.issn.1007-3418.2020.01.016. [12] HOARE M, DAS T, Ageing ALEXANDER G., telomeres, senescence, and injury liver[J]. J Hepatol, 2010, 53( 5): 950- 961. DOI: 10.1016/j.jhep.2010.06.009. [13] TANG CL, CHEN H, JIANG L, et al. Liver regeneration: Changes in oxidative stress, immune system, cytokines, and epigenetic modifications associated with aging[J]. Oxid Med Cell Longev, 2022, 2022: 9018811. DOI: 10.1155/2022/9018811. [14] ALLAIRE M, GILGENKRANTZ H. The aged liver: Beyond cellular senescence[J]. Clin Res Hepatol Gastroenterol, 2020, 44( 1): 6- 11. DOI: 10.1016/j.clinre.2019.07.011. [15] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33( 2): 464- 470. DOI: 10.1053/jhep.2001.22172. [16] LI JQ, LIANG X, YOU SL, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 75( 5): 1104- 1115. DOI: 10.1016/j.jhep.2021.05.026. [17] MITCHELL O, FELDMAN DM, DIAKOW M, et al. The pathophysiology of thrombocytopenia in chronic liver disease[J]. Hepat Med, 2016, 8: 39- 50. DOI: 10.2147/HMER.S74612. [18] XU XW, HOU ZH, XU YY, et al. The dynamic of platelet count as a novel and valuable predictor for 90-day survival of hepatitis B virus-related acute-on-chronic liver failure patients[J]. Clin Res Hepatol Gastroenterol, 2021, 45( 2): 101482. DOI: 10.1016/j.clinre.2020.06.008. [19] TU Y, LI X, CHEN MJ, et al. Value of platelet count and related scoring models in predicting the prognosis of hepatitis B virus-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2023, 39( 6): 1308- 1312. DOI: 10.3969/j.issn.1001-5256.2023.06.009.涂颖, 李雪, 陈美娟, 等. 血小板计数及相关评分模型对HBV相关慢加急性肝衰竭预后的预测价值[J]. 临床肝胆病杂志, 2023, 39( 6): 1308- 1312. DOI: 10.3969/j.issn.1001-5256.2023.06.009. [20] ZACCHERINI G, WEISS E, MOREAU R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment[J]. JHEP Rep, 2021, 3( 1): 100176. DOI: 10.1016/j.jhepr.2020.100176. [21] JALAN R, SALIBA F, PAVESI M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure[J]. J Hepatol, 2014, 61( 5): 1038- 1047. DOI: 10.1016/j.jhep.2014.06.012. [22] TRIANTAFYLLOU E, WOOLLARD KJ, MCPHAIL MJW, et al. The role of monocytes and macrophages in acute and acute-on-chronic liver failure[J]. Front Immunol, 2018, 9: 2948. DOI: 10.3389/fimmu.2018.02948. -



 PDF下载 ( 1398 KB)
PDF下载 ( 1398 KB)

 下载:
下载: