卡瑞利珠单抗联合分子靶向药物治疗老年晚期肝细胞癌患者的效果和安全性分析
DOI: 10.12449/JCH241017
Efficacy and safety of camrelizumab monoclonal antibody combined with molecular-targeted therapy in elderly patients with advanced hepatocellular carcinoma
-
摘要:
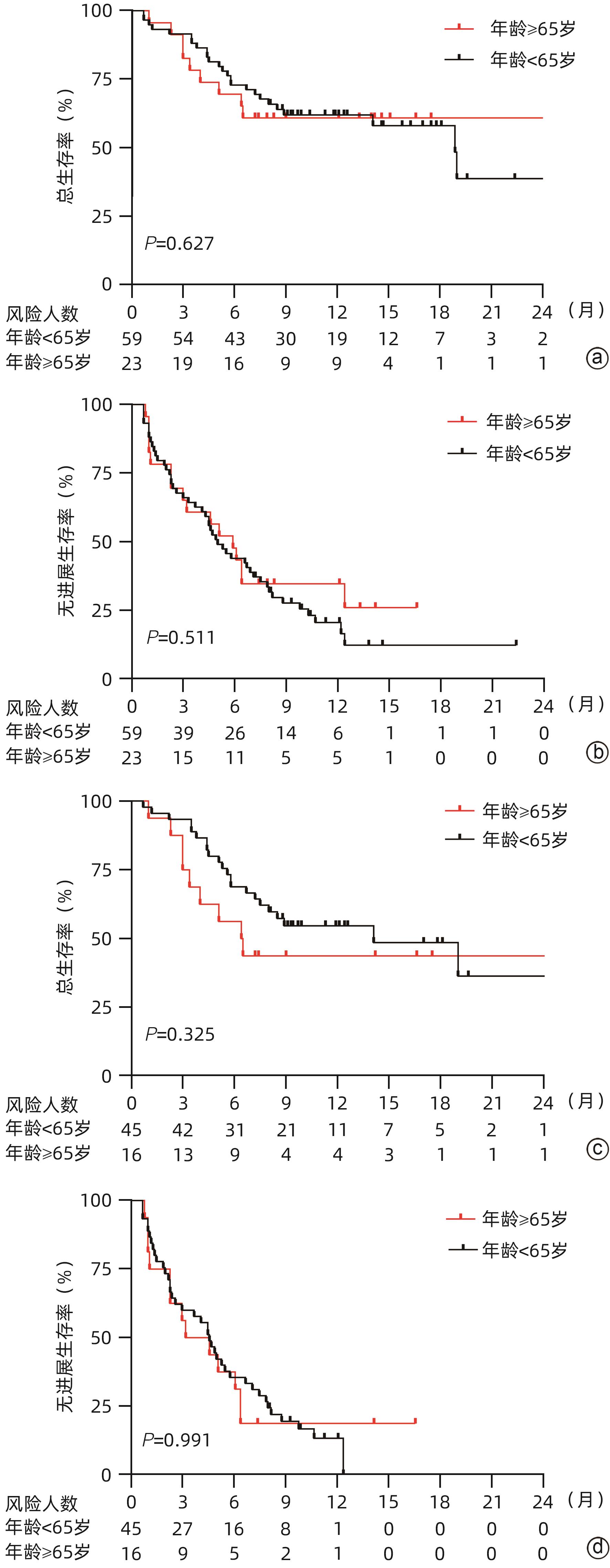
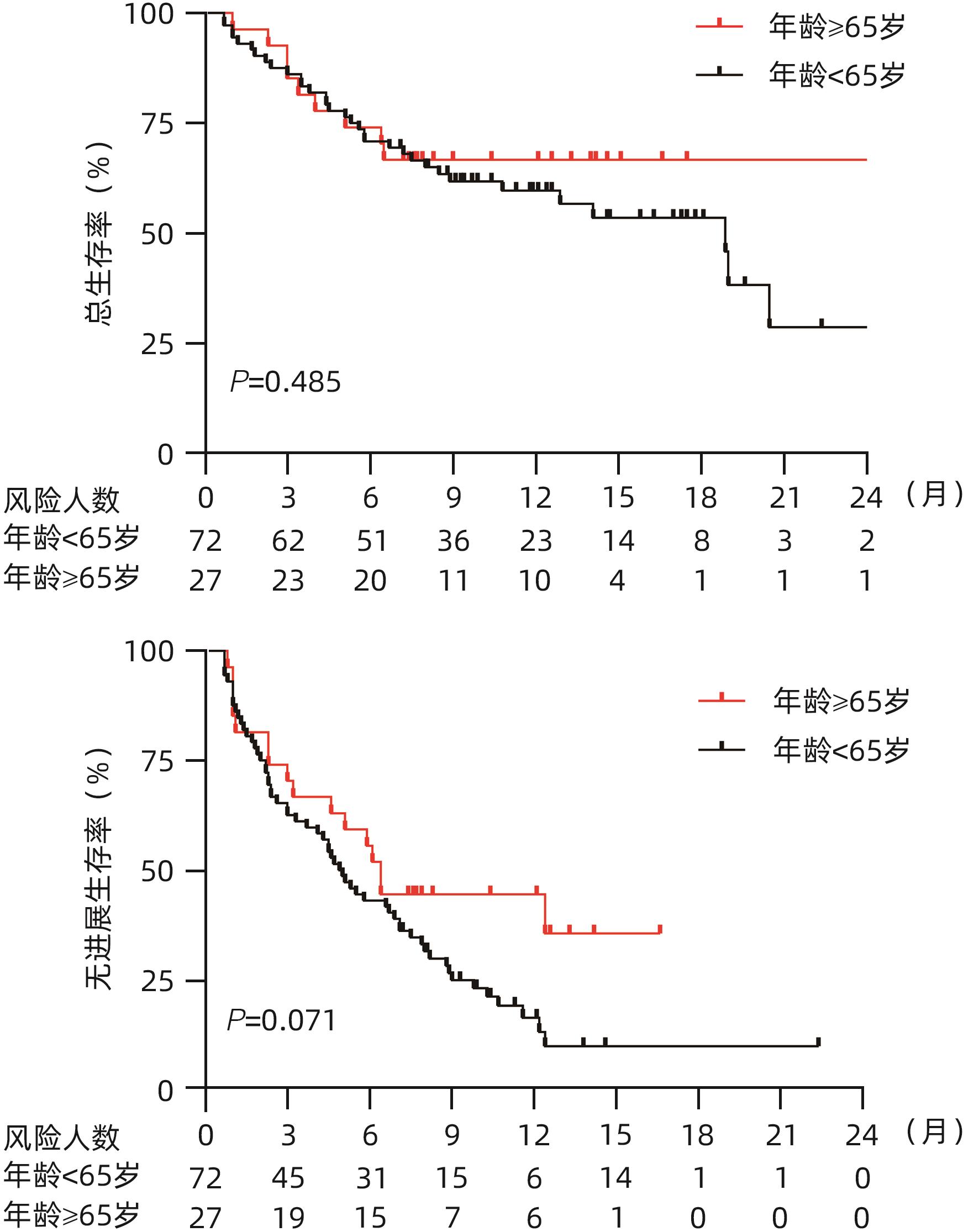
目的 探究老年不可切除/晚期肝细胞癌(HCC)患者使用卡瑞利珠单抗联合分子靶向药物的效果并评估使用过程中的安全情况。 方法 回顾性纳入了2019年1月1日—2021年3月31日就诊于6家医院的不可切除/晚期HCC患者,所有患者接受卡瑞利珠单抗治疗,84.8%的患者同时联合了靶向治疗,根据患者年龄分为老年组(≥65岁)及非老年组(<65岁),评估两组患者总生存期(OS)、无进展生存期(PFS)、客观缓解率(ORR)、疾病控制率(DCR)和免疫相关不良反应(irAE)发生情况。计数资料两组间比较采用χ2检验或Fisher确切检验,符合正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验。生存分析采用Kaplan-Meier法,生存曲线差异比较采用Log-rank检验。采用单因素和多因素Cox比例风险回归分析确定6个月PFS和DCR的独立影响因素。 结果 共纳入99例患者,其中老年组27例,非老年组72例。老年组12个月总生存率、ORR和DCR分别为67.8%、44.4%和74.1%,中位PFS为6.4(3.0~12.4)个月,与非老年组患者比较差异均无统计学意义(P值均>0.05)。老年组的中位OS尚未达到,非老年组中位OS为18.9(13.0~24.8)个月,两组比较差异无统计学意义(P=0.485)。单因素及多因素Cox回归分析显示,基线大血管浸润(MVI)是6个月PFS(HR=2.603,95%CI:1.136~5.964,P=0.024)和DCR(HR=3.963,95%CI:1.671~9.397,P=0.002)的独立危险因素,而年龄、性别、HBV感染病因、出现肝外转移、Child-Pugh B级和AFP>400 ng/mL与6个月PFS和DCR无关。老年组患者中任何级别的irAE和3/4级irAE发生率分别为51.9%和25.9%,与非老年组患者比较差异均无统计学意义(P值均>0.05);两组最常见的irAE为皮肤疾病(39.4%)。 结论 卡瑞利珠单抗联合分子靶向药物治疗≥65岁不可切除/晚期HCC患者的效果和安全性与<65岁患者相当。MVI与免疫治疗应答不佳和不良预后相关。 -
关键词:
- 癌, 肝细胞 /
- 抗体, 单克隆, 人 /
- 分子靶向治疗 /
- 老年人
Abstract:Objective To investigate the efficacy and safety of camrelizumab monoclonal antibody combined with molecular-targeted therapy in elderly patients with unresectable or advanced hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for the patients with unresectable/advanced HCC who attended six hospitals from January 1, 2019 to March 31, 2021, and all patients received camrelizumab monoclonal antibody treatment, among whom 84.8% also received targeted therapy. According to the age of the patients, they were divided into elderly group (≥65 years) and non-elderly group (<65 years). The two groups were assessed in terms of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and immune-related adverse events (irAE). The chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups; the independent samples t-test was used for comparison of normally distributed continuous data, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used for comparison of survival curves. Univariate and multivariate Cox proportional hazards regression analyses were used to determine the independent influencing factors for PFS and DCR at 6 months. Results A total of 99 HCC patients were enrolled, with 27 in the elderly group and 72 in the non-elderly group. The elderly group had an OS rate of 67.8%, an ORR of 44.4%, and a DCR of 74.1% at 12 months and a median PFS of 6.4 (95% confidence interval [CI]: 3.0 — 12.4) months, with no significant differences compared with the non-elderly group (all P>0.05). The median OS was unavailable for the elderly group, while the non-elderly group had an OS of 18.9 (95%CI: 13.0 — 24.8) months; there was no significant difference between the two groups (P=0.485). The univariate and multivariate Cox regression analyses showed that major vascular invasion (MVI) was an independent risk factor for PFS (hazard ratio [HR]=2.603, 95%CI: 1.136 — 5.964, P=0.024) and DCR (HR=3.963, 95%CI: 1.671 — 9.397, P=0.002) at 6 months, while age, sex, etiology of HBV infection, presence of extrahepatic metastasis, Child-Pugh class B, and alpha-fetoprotein>400 ng/mL were not associated with PFS or DCR at 6 months. For the elderly group, the incidence rates of any irAE and grade 3/4 irAE were 51.9% and 25.9%, respectively, with no significant differences compared with the non-elderly group (P>0.05), and skin disease was the most common irAE in both groups (39.4%). Conclusion Camrelizumab monoclonal antibody combined with molecular-targeted therapy has similar efficacy and safety in patients with unresectable/advanced HCC aged ≥65 years and those aged <65 years. MVI is associated with suboptimal response to immunotherapy and poor prognosis. -
表 1 老年组与非老年组患者基线资料比较
Table 1. Baseline characteristic of elderly patiens and non-elderly patients groups
项目 所有患者(n=99) 非老年组(n=72) 老年组(n=27) 统计值 P 值 性别[例(%)] χ2=0.666 0.415 男 82(82.8) 61(84.7) 21(77.8) 女 17(17.2) 11(15.3) 6(22.2) 病因[例(%)] χ2=24.637 <0.001 HBV感染 79(79.8) 65(90.3) 14(51.9) HCV感染 7(7.1) 0(0.0) 7(25.9) 其他 13(13.1) 7(9.7) 6(22.2) AFP[例(%)] χ2=1.071 0.301 ≥400 ng/mL 43(43.4) 29(40.3) 14(51.9) <400 ng/mL 56(56.6) 43(59.7) 13(48.1) Child-Pugh分级[例(%)] χ2=0.694 0.405 A级 58(58.6) 44(61.1) 14(51.9) B级 41(41.4) 28(38.9) 13(48.1) ALBI分级[例(%)] χ2=4.466 0.107 1级 18(18.2) 16(22.2) 2(7.4) 2级 74(74.7) 50(69.4) 24(88.9) 3级 7(7.1) 6(8.3) 1(3.7) BCLC分期[例(%)] χ2=0.542 0.762 A期 17(17.2) 13(18.1) 4(14.8) B期 21(21.2) 14(19.4) 7(25.9) C期 61(61.6) 45(62.5) 16(59.3) MVI[例(%)] χ2=0.175 0.676 无 59(59.6) 42(58.3) 17(63.0) 有 40(40.4) 30(41.7) 10(37.0) 肝外转移[例(%)] χ2=0.175 0.676 无 59(59.6) 42(58.3) 17(63.0) 有 40(40.4) 30(41.7) 10(37.0) 既往治疗[例(%)] χ2=0.047 0.828 无 17(17.2) 12(16.7) 5(18.5) 经导管动脉化疗栓塞 73(73.7) 52(72.2) 21(77.8) 外科手术 21(21.2) 19(26.4) 2(7.4) 射频消融 35(35.4) 28(38.9) 7(25.9) 放疗 5(5.1) 4(5.6) 1(3.7) 靶向药物治疗[例(%)] χ2=0.327 0.567 无 15(15.2) 10(13.9) 5(18.5) 有 84(84.8) 62(86.1) 22(81.5) ECOG评分[例(%)] χ2=3.215 0.360 0分 22(22.2) 17(23.6) 5(18.5) 1分 58(58.6) 43(59.7) 15(55.6) 2分 19(19.2) 12(16.7) 7(25.9) 实验室检查指标(治疗前1个月内) WBC(×109/L) 4.1(1.0~17.5) 4.1(1.0~13.2) 4.3(2.0~17.5) Z=-0.203 0.839 Hb(g/L) 125.28±21.91 125.93±22.09 123.56±22.18 t=0.475 0.636 PLT(×109/L) 111(30~437) 100(30~437) 116(34~275) Z=-0.056 0.573 PT(s) 12.3(9.5~16.8) 12.7(10.3~16.8) 12.2(9.5~16.8) Z=-0.546 0.524 ALT(U/L) 40.9(24.2~62.7) 36.9(23.5~58.0) 56.9(25.0~75.5) Z=-1.658 0.039 AST(U/L) 55.0(35.8~94.2) 53.0(33.9~91.3) 61.2(38.9~111.8) Z=-1.045 0.878 Alb(g/L) 35.1(31.2~38.8) 35.1(31.0~38.3) 34.8(32.3~40.2) Z=-0.656 0.604 TBil(μmol/L) 17.8(4.8~108.2) 17.5(4.8~108.2) 24.3(7.9~69.8) Z=-0.453 0.930 表 2 卡瑞利珠单抗联合治疗后mRESIST疗效评价
Table 2. Evaluation of mRESIST efficacy after carrilizumab combination therapy
疗效评价 所有患者(n=99) 非老年组(n=72) 老年组(n=27) CR[例(%)] 7(7.1) 3(4.2) 4(14.8) PR[例(%)] 29(29.3) 21(29.2) 8(29.6) SD[例(%)] 31(31.3) 23(31.9) 8(29.6) PD[例(%)] 32(32.3) 25(34.7) 7(25.9) ORR[例(%)] 36(36.4) 24(33.3) 12(44.4) DCR[例(%)] 67(67.7) 47(65.3) 20(74.1) 中位PFS(月) 5.3(4.3~6.9) 4.9(3.3~6.9) 6.4(3.0~12.4) 6个月PFS(%) 45.5 50.7 55.3 12个月PFS(%) 20.7 16.3 43.3 中位OS(月) 18.9(12.9~20.5) 18.9(13.0~24.8) NA 6个月OS(%) 71.7 70.9 74.2 12个月OS(%) 61.3 60.0 67.8 注:NA,尚未达中位OS时间。
表 3 6个月PFS相关单因素及多因素Cox风险回归分析
Table 3. Univariate and multifactorial Cox risk regression analyses of influencing factors associated with 6-month PFS
变量 单变量分析 多变量分析 HR 95%CI P值 HR 95%CI P值 性别(女/男) 1.035 0.331~3.238 0.953 年龄(<65岁/≥65岁) 0.529 0.177~1.580 0.254 病因(HBV感染/非HBV感染) 1.450 0.402~5.232 0.571 AFP(≤400 ng/mL/>400 ng/mL) 2.032 0.824~5.012 0.124 BCLC 分期(A/B/C) 0.631 0.247~1.614 0.337 MVI(是/否) 3.732 1.075~12.957 0.038 2.603 1.136~5.964 0.024 肝外转移(是/否) 2.777 0.773~9.969 0.117 靶向治疗(是/否) 0.755 0.215~2.646 0.660 Child-Pugh分级(A/B) 0.987 0.363~2.685 0.979 ALBI分级(1/2/3) 0.839 0.304~2.316 0.735 表 4 6个月DCR相关单因素及多因素Cox风险回归分析
Table 4. Univariate and multifactorial Cox risk regression analyses of influencing factors associated with 6-month DCR
变量 单变量分析 多变量分析 HR 95%CI P值 HR 95%CI P值 性别(女/男) 0.606 0.159~2.310 0.463 年龄(<65岁/≥65岁) 0.878 0.260~2.972 0.835 病因(HBV感染/非HBV感染) 0.520 0.119~2.271 0.384 AFP(≤400 ng/mL/>400 ng/mL) 1.933 0.735~5.082 0.182 BCLC 分期(A/B/C) 0.366 0.131~1.026 0.056 MVI(是/否) 8.532 2.186~33.302 0.002 3.963 1.671~9.397 0.002 肝外转移(是/否) 2.606 0.722~9.403 0.143 靶向治疗(是/否) 0.528 0.136~2.047 0.355 Child-Pugh分级(A/B) 1.233 0.431~3.530 0.697 ALBI分级(1/2/3) 1.053 0.335~3.308 0.929 表 5 老年组和非老年组患者irAE的发生情况
Table 5. IrAE in elderly and non-elderly patients
项目 任何级别不良反应 ≥3级不良反应 非老年组(n=72) 老年组(n=27) P值 非老年组(n=72) 老年组(n=27) P值 不良反应合计[例(%)] 38(52.8) 14(51.9) 0.917 17(23.6) 7(25.9) 0.764 皮肤疾病[例(%)] 26(36.1) 13(48.1) 0.669 0 0 皮肤瘙痒症 10(13.9) 5(18.5) 0.543 0 0 斑丘疹 8(11.1) 5(18.5) 0.303 0 0 反应性皮肤毛细血管增生症 8(11.1) 3(11.1) 0.988 0 0 内分泌疾病[例(%)] 16(22.2) 9(33.3) 0.320 4(5.6) 5(18.5) 0.113 甲状腺功能减退症 11(15.3) 4(14.8) 0.935 0 0 原发性肾上腺皮质功能减退 1(1.4) 3(11.1) 0.058 1(1.4) 3(11.1) 0.058 垂体炎 2(2.8) 2(7.4) 0.179 2(2.8) 2(7.4) 0.179 甲状腺功能亢进症 2(2.8) 0 >0.05 1(1.4) 0 >0.05 血小板计数降低[例(%)] 22(30.6) 6(22.2) 0.501 8(11.1) 1(3.7) 0.630 肝胆疾病[例(%)] 13(18.1) 3(11.1) 0.659 10(13.9) 3(11.1) 0.918 腹泻[例(%)] 12(16.7) 3(11.1) 0.138 0 1(3.7) 0.200 心脏疾病[例(%)] 4(5.6) 3(11.1) 0.376 1(1.4) 0 >0.05 高血糖症[例(%)] 6(8.3) 1(3.7) 0.657 0 0 肾脏疾病[例(%)] 2(2.8) 1(3.7) >0.05 0 0 呼吸道疾病[例(%)] 1(1.4) 0 >0.05 1(1.4) 0 >0.05 -
[1] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16( 10): 589- 604. DOI: 10.1038/s41575-019-0186-y. [2] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [3] SMITH BD, SMITH GL, HURRIA A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation[J]. J Clin Oncol, 2009, 27( 17): 2758- 2765. DOI: 10.1200/JCO.2008.20.8983. [4] LLOVET JM, KELLEY RK, VILLANUEVA A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7: 6. DOI: 10.1038/s41572-020-00240-3. [5] LIU JH, CHEN ZC, LI YQ, et al. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy[J]. Front Pharmacol, 2021, 12: 731798. DOI: 10.3389/fphar.2021.731798. [6] RAO Q, LI M, XU W, et al. Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: A systematic review and meta-analysis[J]. Hepatol Int, 2020, 14( 5): 765- 775. DOI: 10.1007/s12072-020-10064-8. [7] WANG Y, JIANG M, ZHU JJ, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma[J]. Biomedecine Pharmacother, 2020, 132: 110797. DOI: 10.1016/j.biopha.2020.110797. [8] FINN RS, IKEDA M, ZHU AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38( 26): 2960- 2970. DOI: 10.1200/JCO.20.00808. [9] OMATA M, CHENG AL, KOKUDO N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update[J]. Hepatol Int, 2017, 11( 4): 317- 370. DOI: 10.1007/s12072-017-9799-9. [10] LI T, GUO J, LIU YS, et al. Effectiveness and tolerability of camrelizumab combined with molecular targeted therapy for patients with unresectable or advanced HCC[J]. Cancer Immunol Immunother, 2023, 72( 7): 2137- 2149. DOI: 10.1007/s00262-023-03404-8. [11] LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72( 2): 288- 306. DOI: 10.1016/j.jhep.2019.09.026. [12] MEI KM, QIN SK, CHEN ZD, et al. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: Cohort A report in a multicenter phase Ib/II trial[J]. J Immunother Cancer, 2021, 9( 3): e002191. DOI: 10.1136/jitc-2020-002191. [13] CAI JQ, ZHANG BL, BI XY. Comprehensive treatment strategy for hepatocellular carcinoma based on surgical treatment in the era of targeted therapy and immunotherapy[J]. Chin J Dig Surg, 2023, 22( 2): 181- 186. DOI: 10.3760/cma.j.cn115610-20221130-0071.蔡建强, 张搏伦, 毕新宇. 靶向联合免疫治疗时代以外科为主的肝细胞癌综合治疗策略[J]. 中华消化外科杂志, 2023, 22( 2): 181- 186. DOI: 10.3760/cma.j.cn115610-20221130-00719. [14] LI H, QIN SK, LIU Y, et al. Camrelizumab combined with FOLFOX4 regimen as first-line therapy for advanced hepatocellular carcinomas: A sub-cohort of a multicenter phase ib/II study[J]. Drug Des Devel Ther, 2021, 15: 1873- 1882. DOI: 10.2147/DDDT.S304857. [15] YUAN GS, CHENG X, LI Q, et al. Safety and efficacy of camrelizumab combined with apatinib for advanced hepatocellular carcinoma with portal vein tumor Thrombus: A multicenter retrospective study[J]. Onco Targets Ther, 2020, 13: 12683- 12693. DOI: 10.2147/OTT.S286169. [16] QIN SK, REN ZG, MENG ZQ, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21( 4): 571- 580. DOI: 10.1016/S1470-2045(20)30011-5. [17] XU JM, SHEN J, GU SZ, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma(RESCUE): A nonrandomized, open-label, phase II trial[J]. Clin Cancer Res, 2021, 27( 4): 1003- 1011. DOI: 10.1158/1078-0432.CCR-20-2571. [18] VITHAYATHIL M, D’ALESSIO A, FULGENZI CAM, et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma[J]. Liver Int, 2022, 42( 11): 2538- 2547. DOI: 10.1111/liv.15405. [19] TADA T, KUMADA T, HIRAOKA A, et al. Safety and efficacy of atezolizumab plus bevacizumab in elderly patients with hepatocellular carcinoma: A multicenter analysis[J]. Cancer Med, 2022, 11( 20): 3796- 3808. DOI: 10.1002/cam4.4763. [20] BORZIO M, DIONIGI E, VITALE A, et al. Management and prognosis of hepatocellular carcinoma in the elderly: Results of an in-field multicenter cohort study[J]. Liver Int, 2017, 37( 8): 1184- 1192. DOI: 10.1111/liv.13392. [21] GUO H, WU T, LU Q, et al. Hepatocellular carcinoma in elderly: Clinical characteristics, treatments and outcomes compared with younger adults[J]. PLoS One, 2017, 12( 9): e0184160. DOI: 10.1371/journal.pone.0184160. [22] DELIGIORGI MV, TRAFALIS DT. Reversible primary adrenal insufficiency related to anti-programmed cell-death 1 protein active immunotherapy: Insight into an unforeseen outcome of a rare immune-related adverse event[J]. Int Immunopharmacol, 2020, 89( Pt B): 107050. DOI: 10.1016/j.intimp.2020.107050. [23] ZHANG Y, ZHANG X, LI W, et al. Biomarkers and risk factors for the early prediction of immune-related adverse events: a review[J]. Hum Vaccin Immunother, 2022, 18( 1): 2018894. DOI: 10.1080/21645515.2021.2018894. -



 PDF下载 ( 1217 KB)
PDF下载 ( 1217 KB)

 下载:
下载: