叶酸对邻苯二甲酸二(2-乙基己基)酯(DEHP)暴露导致小鼠胆汁淤积型肝损伤的保护作用
DOI: 10.12449/JCH241021
伦理学声明:本研究遵守国家所有相关法规、机构政策和赫尔辛基宣言,于2020年5月23日经由安徽医科大学动物伦理委员会审批,批号:20200523,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:王建青、余芸、侯梦贞负责课题设计,资料分析;侯梦贞、黄倩倩、张伦、陶文康、蒋月参与收集数据,修改论文;王建青负责拟定写作思路,指导撰写文章并最后定稿。
Protective effect of folic acid against cholestatic liver injury in mice caused by bis(2-ethylhexyl) phthalate exposure
-
摘要:
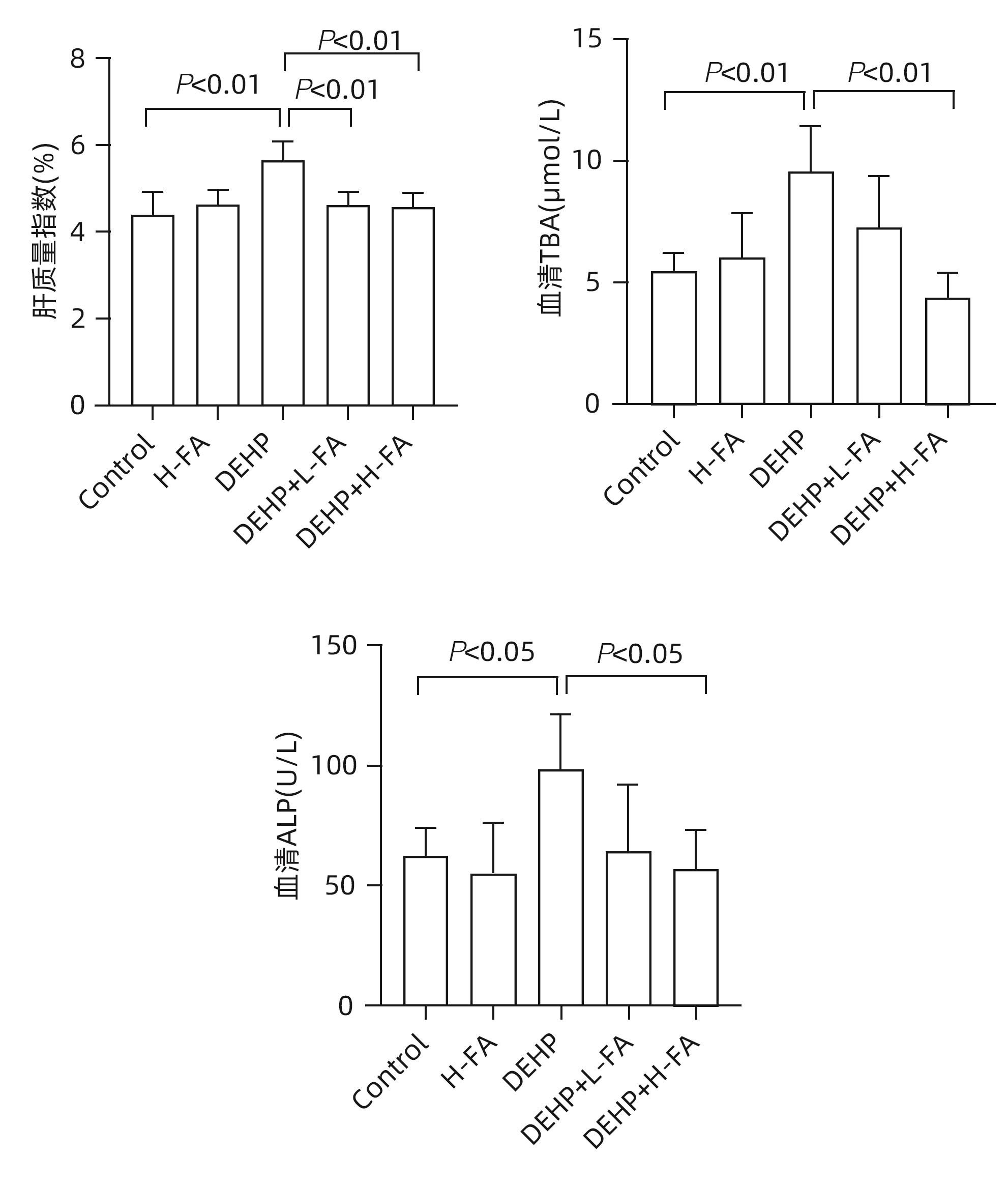
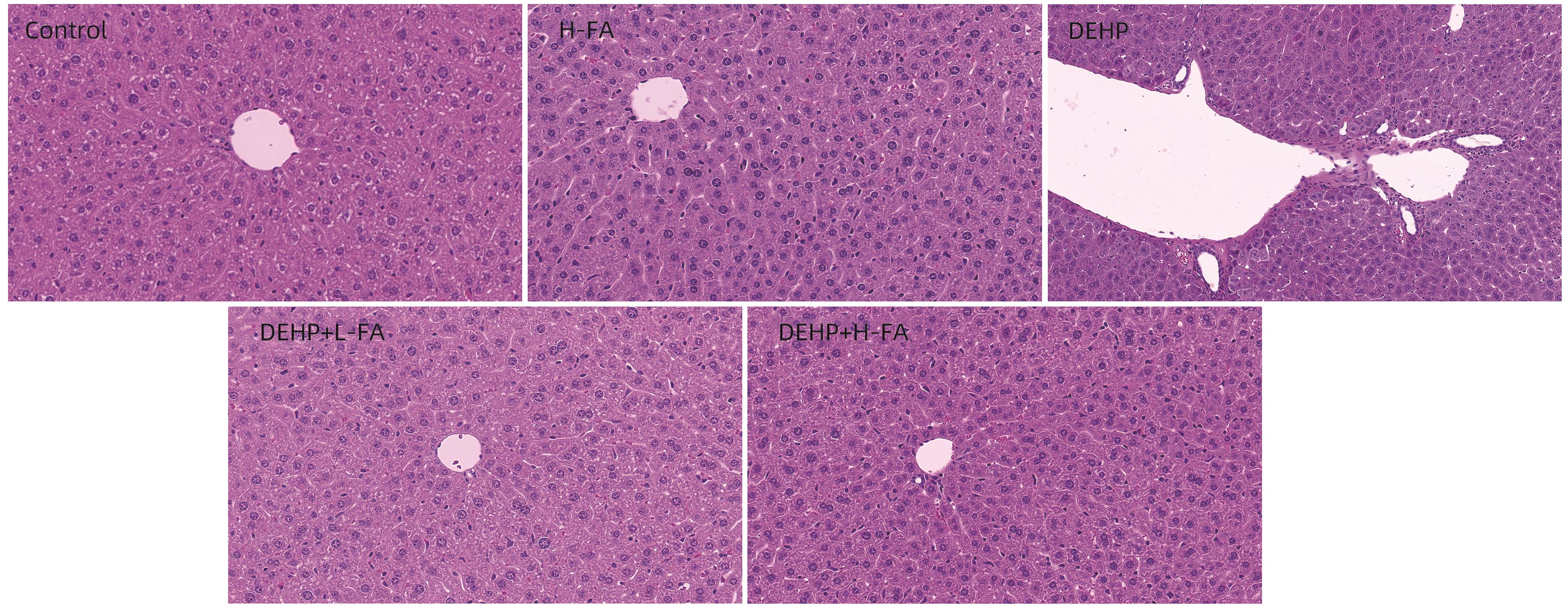
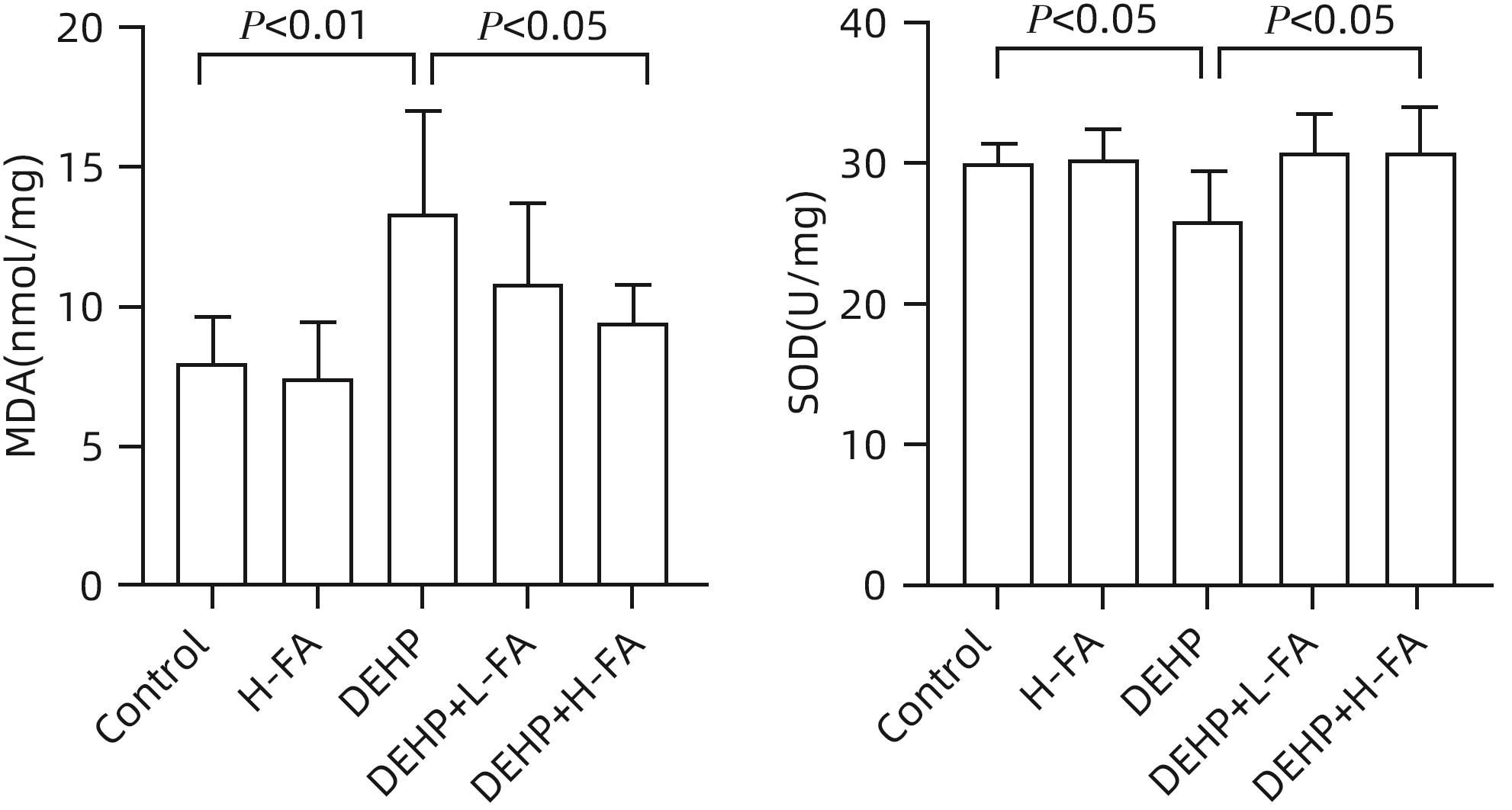
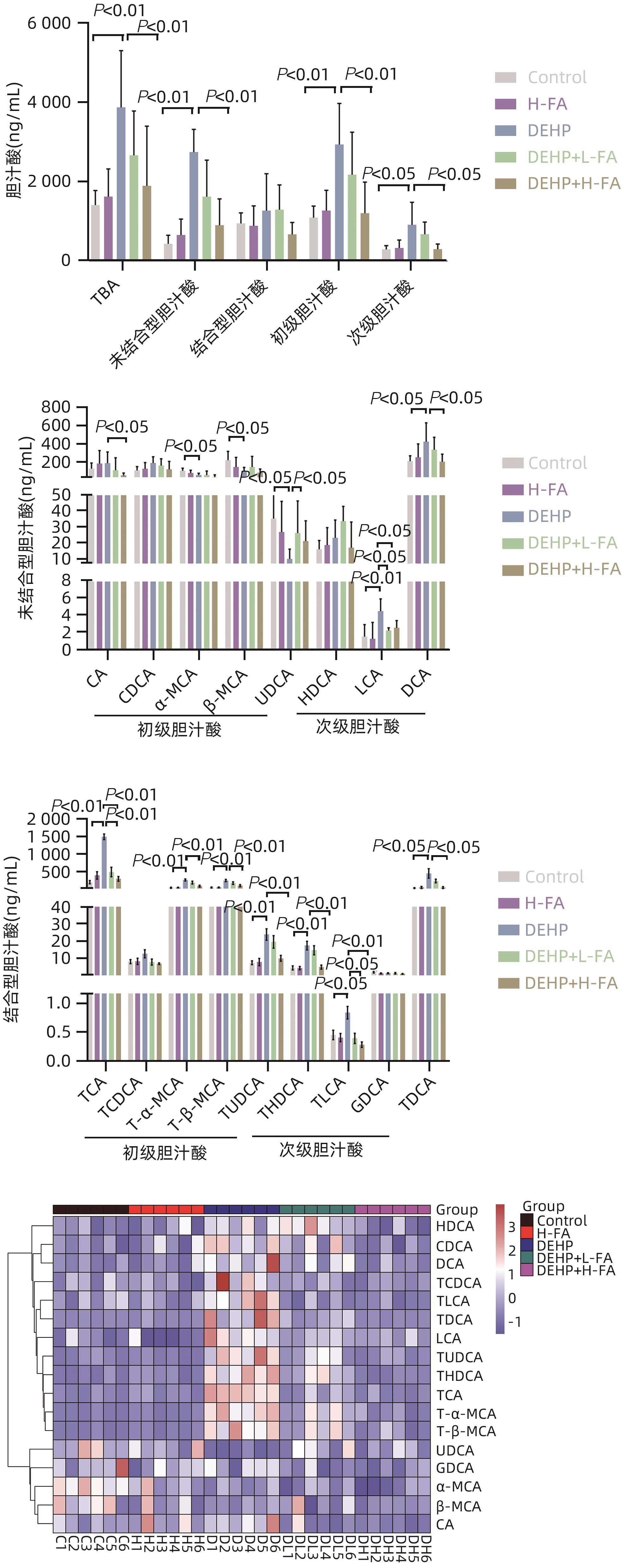
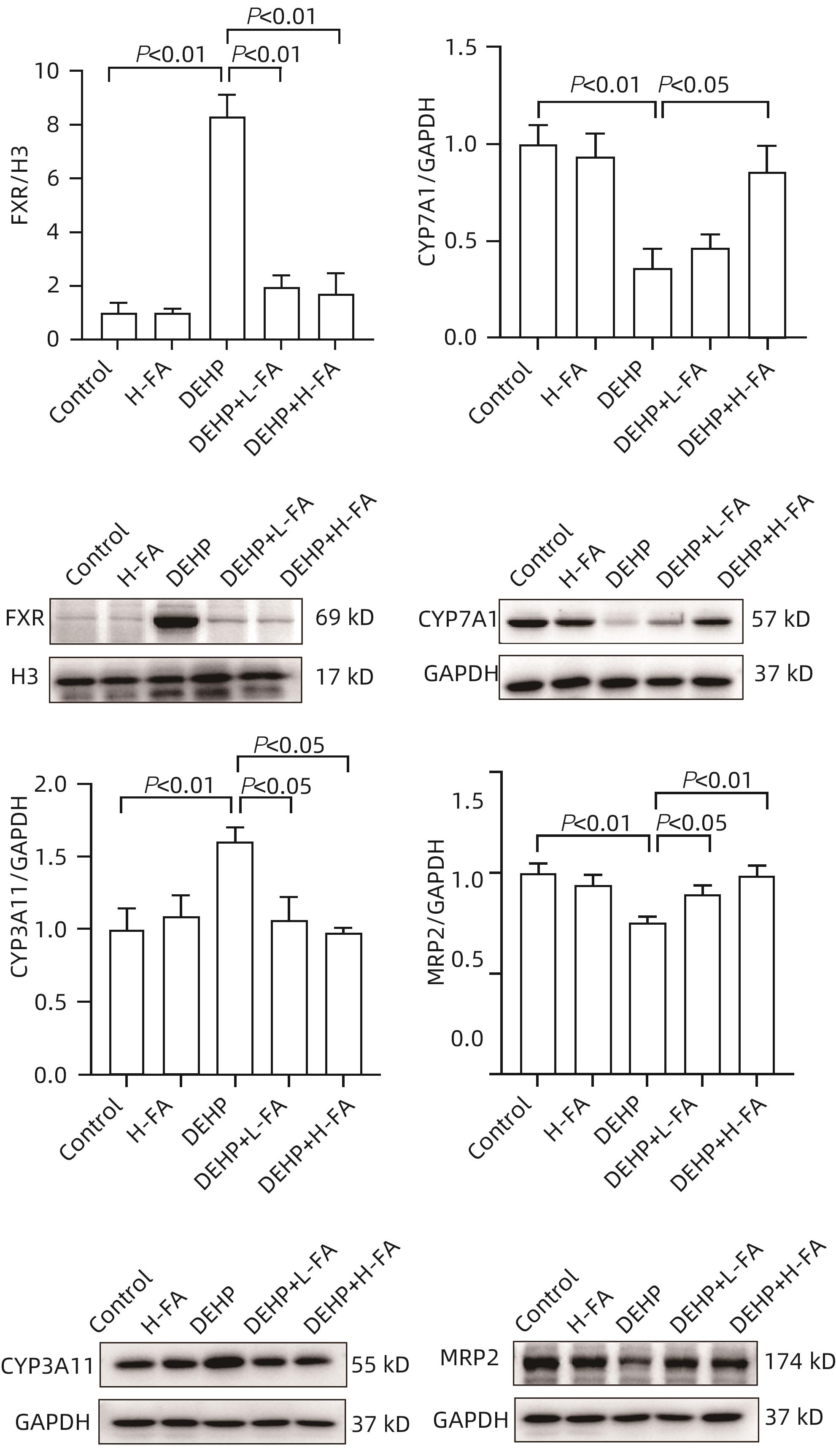
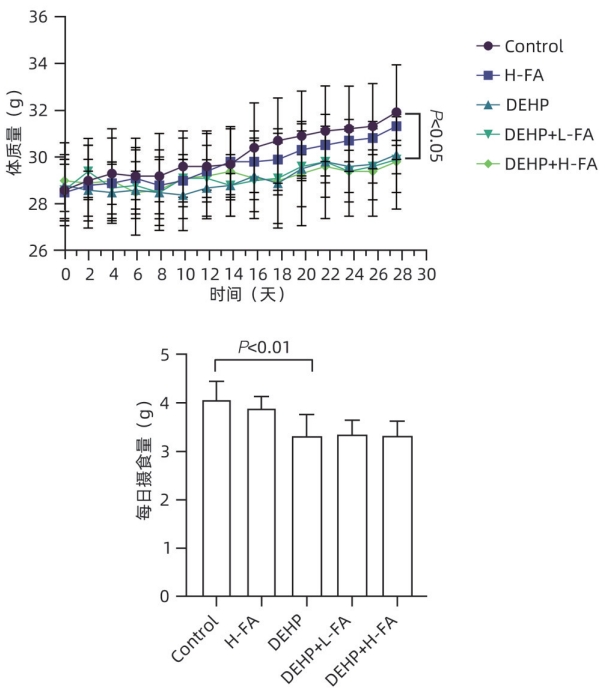
目的 探讨叶酸对邻苯二甲酸二(2-乙基己基)酯(DEHP)暴露诱发小鼠胆汁淤积型肝损伤的保护作用及其机制。 方法 将ICR小鼠随机分为对照(Control)组、叶酸高剂量(H-FA)组、DEHP组、DEHP+叶酸低剂量(DEHP+L-FA)组、DEHP+叶酸高剂量(DEHP+H-FA)组,每组6只。H-FA组、DEHP+L-FA组和DEHP+H-FA组给予相应剂量的叶酸灌胃,Control组和DEHP组灌胃等量的PBS溶液。2 h后,DEHP组、DEHP+L-FA组和DEHP+H-FA组给予含200 mg/kg DEHP的玉米油,Control组和H-FA组灌胃等量的纯玉米油,共灌胃4周。记录小鼠每天的体质量和摄食量,收集血液和肝组织。生化仪检测血清总胆汁酸(TBA)和碱性磷酸酶(ALP)水平;HE染色观察肝组织病理变化;试剂盒检测肝组织丙二醛(MDA)和超氧化物歧化酶(SOD)含量;LC-MS/MS检测小鼠血清胆汁酸谱;Western Blot法检测肝脏胆汁酸代谢相关蛋白的表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与对照组相比,DEHP组小鼠每日摄食量明显下降,体质量从第10天开始显著降低(P值均<0.05);与DEHP组相比,DEHP+L-FA组与DEHP+H-FA组小鼠的体质量和摄食量基本不变(P值均>0.05)。与对照组相比,DEHP组小鼠肝质量指数、血清TBA及ALP均显著升高(P值均<0.05),肝组织可见汇管区扩大,胆管变形增生及少量炎性细胞浸润;与DEHP组相比,DEHP+L-FA组与DEHP+H-FA组小鼠肝质量指数均明显下降(P值均<0.01),DEHP+H-FA组的血清TBA和ALP均显著下降(P值均<0.05),叶酸干预后小鼠肝组织形态结构明显改善。与对照组相比,DEHP组小鼠肝脏SOD含量明显下降(P<0.05),肝脏MDA含量明显增加(P<0.01);与DEHP组相比,MDA和SOD含量在DEHP+H-FA组均显著回调(P值均<0.05)。与对照组相比,DEHP组小鼠血清中α-鼠胆酸(α-MCA)、β-鼠胆酸(β-MCA)、去氧胆酸(DCA)、石胆酸(LCA)、牛磺胆酸(TCA)、牛磺去氧胆酸(TDCA)、牛磺熊去氧胆酸(TUDCA)、牛磺-β-鼠胆酸(T-β-MCA)、牛磺-α-鼠胆酸(T-α-MCA)、牛磺猪去氧胆酸(THDCA)、牛磺石胆酸(TLCA)均明显升高(P值均<0.05),熊去氧胆酸(UDCA)明显降低(P<0.05);与DEHP组相比,血清中DCA、LCA、TCA、TDCA、TUDCA、T-β-MCA、T-α-MCA、THDCA、TLCA在DEHP+H-FA组均明显回调(P值均<0.05)。与对照组相比,DEHP组小鼠肝脏FXR和CYP3A11蛋白表达量均明显增加(P值均<0.01),CYP7A1和MRP2蛋白表达量显著降低(P值均<0.01);与DEHP组相比,肝脏FXR和CYP3A11蛋白表达量在DEHP+L-FA组和DEHP+H-FA组中均明显下调(P值均<0.05);MRP2蛋白表达量在DEHP+L-FA组DEHP+H-FA组中均显著上调(P值均<0.05);CYP7A1蛋白表达量在DEHP+H-FA组中显著上调(P<0.05)。 结论 叶酸对DEHP暴露导致的小鼠胆汁淤积型肝损伤有保护作用,其机制可能是调节胆汁酸合成、代谢与转运从而维持胆汁酸稳态。 -
关键词:
- 邻苯二甲酸二(2-乙基己基)酯 /
- 叶酸 /
- 胆汁淤积 /
- 胆汁酸类
Abstract:Objective To investigate the protective effect of folic acid against cholestatic liver injury in mice induced by bis(2-ethylhexyl) phthalate (DEHP) exposure and its mechanism. Methods ICR mice were randomly divided into control group, high-dose folic acid (H-FA) group, DEHP group, DEHP+low-dose folic acid (DEHP+L-FA) group, and DEHP+high-dose folic acid (DEHP+H-FA) group, with 6 mice in each group. The mice in the H-FA group, the DEHP+L-FA group, and the DEHP+H-FA group were given folic acid by gavage at the corresponding dose, and those in the control group and the DEHP group were given an equal volume of PBS solution by gavage. After 2 hours, the mice in the DEHP group, the DEHP+L-FA group, and the DEHP+H-FA group were given corn oil containing 200 mg/kg DEHP, and those in the control group and the H-FA group were given an equal volume of pure corn oil, by gavage for 4 weeks. Body weight and food intake were recorded every day, and blood and liver tissue samples were collected. A biochemical analyzer was used to measure the serum levels of total bile acid (TBA) and alkaline phosphatase(ALP); HE staining was used to observe the histopathological changes of liver tissue; kits were used to measure the content of malondialdehyde (MDA) and superoxide dismutase (SOD) in the liver; LC-MS/MS was used to measure serum bile acid profiles; Western blot was used to measure the expression levels of proteins associated with hepatic bile acid metabolism. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the control group, the daily food intake of the mice in the DEHP group decreased significantly, and the body weight decreased significantly from day 10 (P<0.05), and compared with the DEHP group, the DEHP+L-FA group and the DEHP+H-FA group had basically unchanged body weight and daily food intake (P>0.05). Compared with the control group, the DEHP group had significant increases in liver weight index and the serum levels of TBA and ALP (all P<0.05), with enlarged portal area, bile duct deformity and hyperplasia, and a small amount of inflammatory cell infiltration in liver tissue; compared with the DEHP group, the DEHP+L-FA group and the DEHP+H-FA group had a significant reduction in liver weight index (P<0.01), and the DEHP+H-FA group had significant reductions in the serum levels of TBA and ALP (P<0.05), with a significant improvement in liver histomorphology and structure after folic acid intervention. Compared with the control group, the DEHP group had a significant reduction in the content of SOD (P<0.05) and a significant increase in the content of MDA in the liver (P<0.01), and compared with the DEHP group, the DEHP+H-FA group had significant reductions in the content of MDA and SOD (P<0.05). Compared with the control group, the DEHP group had significant increases in the serum levels of α-muricholic acid (α-MCA),β- muricholic acid (β-MCA),deoxycholic acid (DCA), lithocholic acid (LCA), taurocholic acid (TCA), taurodeoxycholic acid (TDCA), tauroursodeoxycholic acid (TUDCA), tauro-β-muricholic acid (T-β-MCA), tauro-α-muricholic acid (T-α-MCA), taurohyodeoxycholic acid (THDCA), and taurolithocholic acid (TLCA) (P<0.05) and a significant reduction in ursodeoxycholic acid (UDCA)(P<0.05); compared with the DEHP group, the DEHP+H-FA group had significant reductions in the serum levels of DCA, LCA, TCA, TDCA, TUDCA, T-β-MCA, T-α-MCA, THDCA, and TLCA (P<0.05). Compared with the control group, the DEHP group had significant increases in the protein expression levels of FXR and CYP3A11 in the liver (P<0.01) and significant reductions in the protein expression levels of CYP7A1 and MRP2 (P<0.01); compared with the DEHP group, the DEHP+L-FA group and the DEHP+H-FA group had significant reductions in the protein expression levels of FXR and CYP3A11 in the liver (P<0.05) and a significant increase in the protein expression level of MRP2 (P<0.05), and the DEHP+H-FA group had a significant increase in the protein expression level of CYP7A1 (P<0.05). Conclusion Folic acid has a protective effect against cholestatic liver injury in mice induced by DEHP exposure, possibly by regulating bile acid synthesis, catabolism, and transport and maintaining bile acid homeostasis. -
Key words:
- Di-(2-Ethylhexyl) Phthalate /
- Folic Acid /
- Cholestasis /
- Bile Acid
-
-
[1] BAGEL S, DESSAIGNE B, BOURDEAUX D, et al. Influence of lipid type on bis(2-ethylhexyl)phthalate(DEHP) leaching from infusion line sets in parenteral nutrition[J]. JPEN J Parenter Enteral Nutr, 2011, 35( 6): 770- 775. DOI: 10.1177/0148607111414021. [2] ZHAO F, ZHANG L, QU MC, et al. Obeticholic acid alleviates intrauterine growth restriction induced by di-ethyl-hexyl phthalate in pregnant female mice by improving bile acid disorder[J]. Environ Sci Pollut Res Int, 2023, 30( 51): 110956- 110969. DOI: 10.1007/s11356-023-30149-9. [3] GALLEGO-LOPEZ MDC, OJEDA ML, ROMERO-HERRERA I, et al. Folic acid homeostasis and its pathways related to hepatic oxidation in adolescent rats exposed to binge drinking[J]. Antioxidants(Basel), 2022, 11( 2): 362. DOI: 10.3390/antiox11020362. [4] ZHANG HQ, ZUO YW, ZHAO HC, et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis[J]. Front Nutr, 2022, 9: 989311. DOI: 10.3389/fnut.2022.989311. [5] CHEN S, YANG MY, WANG R, et al. Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota[J]. Eur J Nutr, 2022, 61( 4): 2015- 2031. DOI: 10.1007/s00394-021-02769-9. [6] JIANG L, GAI XC, NI Y, et al. Folic acid protects against tuberculosis-drug-induced liver injury in rats and its potential mechanism by metabolomics[J]. J Nutr Biochem, 2023, 112: 109214. DOI: 10.1016/j.jnutbio.2022.109214. [7] ZHANG YJ, GUO JL, XUE JC, et al. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment[J]. Environ Pollut, 2021, 291: 118106. DOI: 10.1016/j.envpol.2021.118106. [8] GAITANTZI H, HAKENBERG P, THEOBALD J, et al. Di(2-ethylhexyl) phthalate and its role in developing cholestasis: An in vitro study on different liver cell types[J]. J Pediatr Gastroenterol Nutr, 2018, 66( 2): e28- e35. DOI: 10.1097/MPG.0000000000001813. [9] WEI XJ, YANG DQ, ZHANG BY, et al. Di-(2-ethylhexyl) phthalate increases plasma glucose and induces lipid metabolic disorders via FoxO1 in adult mice[J]. Sci Total Environ, 2022, 842: 156815. DOI: 10.1016/j.scitotenv.2022.156815. [10] ZHOU YH, ZHOU YZ, LI YF, et al. Targeted bile acid profiles reveal the liver injury amelioration of Da-Chai-Hu Decoction against ANIT- and BDL-induced cholestasis[J]. Front Pharmacol, 2022, 13: 959074. DOI: 10.3389/fphar.2022.959074. [11] WANG GF, LI YY, SHI R, et al. Yinchenzhufu decoction protects against alpha-naphthylisothiocyanate-induced acute cholestatic liver injury in mice by ameliorating disordered bile acid homeostasis and inhibiting inflammatory responses[J]. J Ethnopharmacol, 2020, 254: 112672. DOI: 10.1016/j.jep.2020.112672. [12] LE YB, WANG KH, ZOU L. Mechanism of taurocholic acid in promoting the progression of liver cirrhosis[J]. J Clin Hepatol, 2021, 37( 11): 2658- 2662. DOI: 10.3969/j.issn.1001-5256.2021.11.037.乐英彪, 王昆华, 邹雷. 牛磺胆酸促进肝硬化发展的机制[J]. 临床肝胆病杂志, 2021, 37( 11): 2658- 2662. DOI: 10.3969/j.issn.1001-5256.2021.11.037. [13] LI CZ, HUANG XW, ZHANG ZP, et al. Research progress in role of gut-liver axis in occurrence and development of atherosclerosis[J]. J Jilin Univ Med Ed, 2023, 49( 6): 1669- 1676. DOI: 10.13481/j.1671-587X.20230636.李朝政, 黄晓巍, 张泽鹏, 等. 肠-肝轴在动脉粥样硬化发生发展中作用的研究进展[J]. 吉林大学学报(医学版), 2023, 49( 6): 1669- 1676. DOI: 10.13481/j.1671-587X.20230636. [14] TRAUNER M, FUCHS CD. Novel therapeutic targets for cholestatic and fatty liver disease[J]. Gut, 2022, 71( 1): 194- 209. DOI: 10.1136/gutjnl-2021-324305. [15] HAN X, LIN C, LIU H, et al. Allocholic acid protects against α- naphthylisothiocyanate-induced cholestasis in mice by ameliorating disordered bile acid homeostasis[J]. J Appl Toxicol, 2024, 44( 4): 582- 594. DOI: 10.1002/jat.4562. [16] CHAI J, CAI SY, LIU XC, et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis[J]. J Hepatol, 2015, 63( 6): 1440- 1448. DOI: 10.1016/j.jhep.2015.07.016. [17] PAULUSMA CC, KOTHE MJ, BAKKER CT, et al. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver[J]. Hepatology, 2000, 31( 3): 684- 693. DOI: 10.1002/hep.510310319. [18] ZU Y, LIU YN, LAN LL, et al. Consecutive baicalin treatment relieves its accumulation in rats with intrahepatic cholestasis by increasing MRP2 expression[J]. Heliyon, 2023, 9( 1): e12689. DOI: 10.1016/j.heliyon.2022.e12689. [19] RAZORI MV, MAIDAGAN PM, CIRIACI N, et al. Anticholestatic mechanisms of ursodeoxycholic acid in lipopolysaccharide-induced cholestasis[J]. Biochem Pharmacol, 2019, 168: 48- 56. DOI: 10.1016/j.bcp.2019.06.009. [20] HINOSHITA E, TAGUCHI K, INOKUCHI A, et al. Decreased expression of an ATP-binding cassette transporter, MRP2, in human livers with hepatitis C virus infection[J]. J Hepatol, 2001, 35( 6): 765- 773. DOI: 10.1016/s0168-8278(01)00216-1. -



 PDF下载 ( 3319 KB)
PDF下载 ( 3319 KB)

 下载:
下载: