慢性HBV感染者组织学免疫耐受状态无创诊断指标及相关模型的建立与评价
DOI: 10.12449/JCH241208
Noninvasive diagnostic indicators for histologically defined immune tolerance state in patients with chronic HBV infection and establishment and assessment of related models
-
摘要:
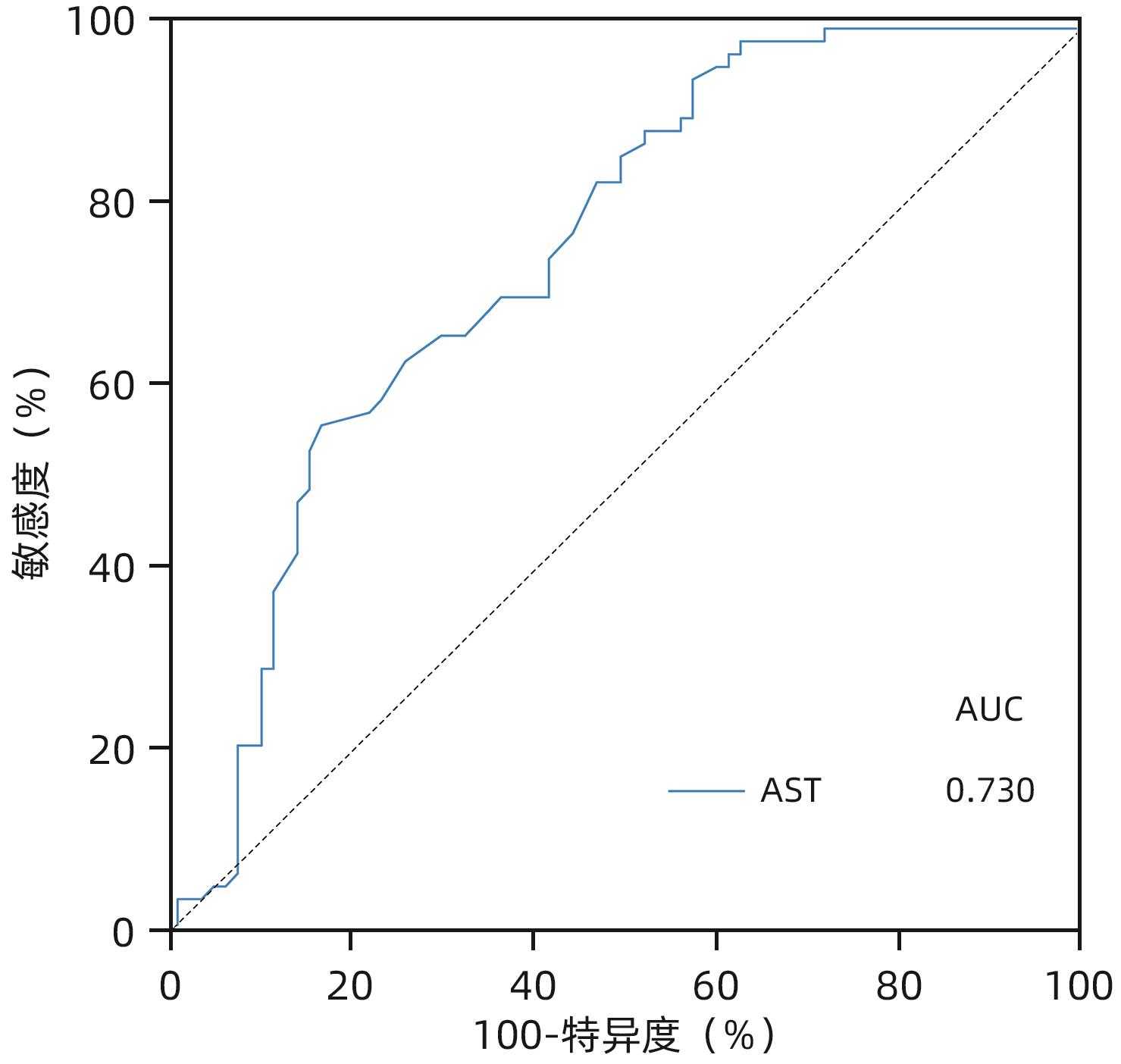
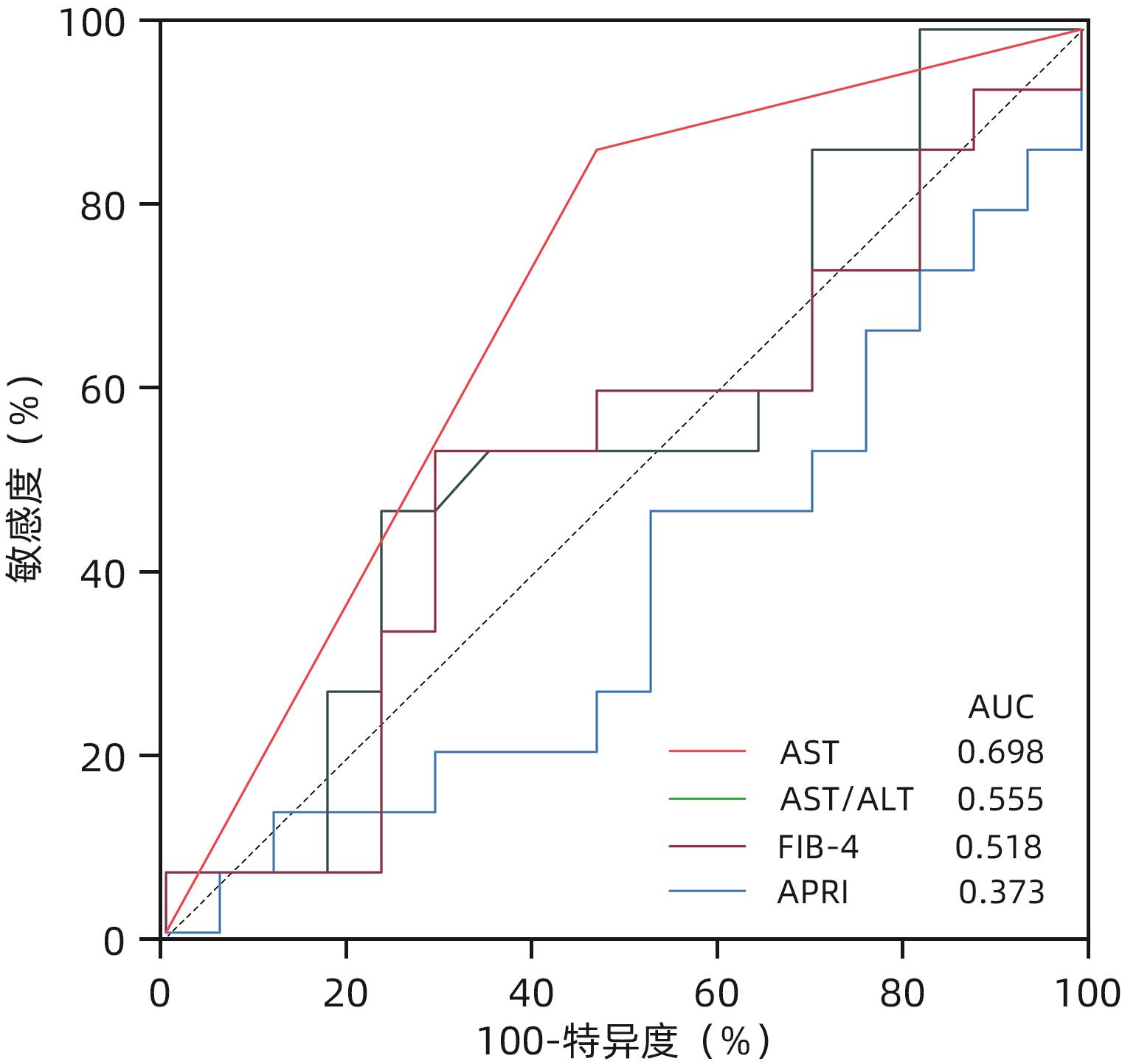
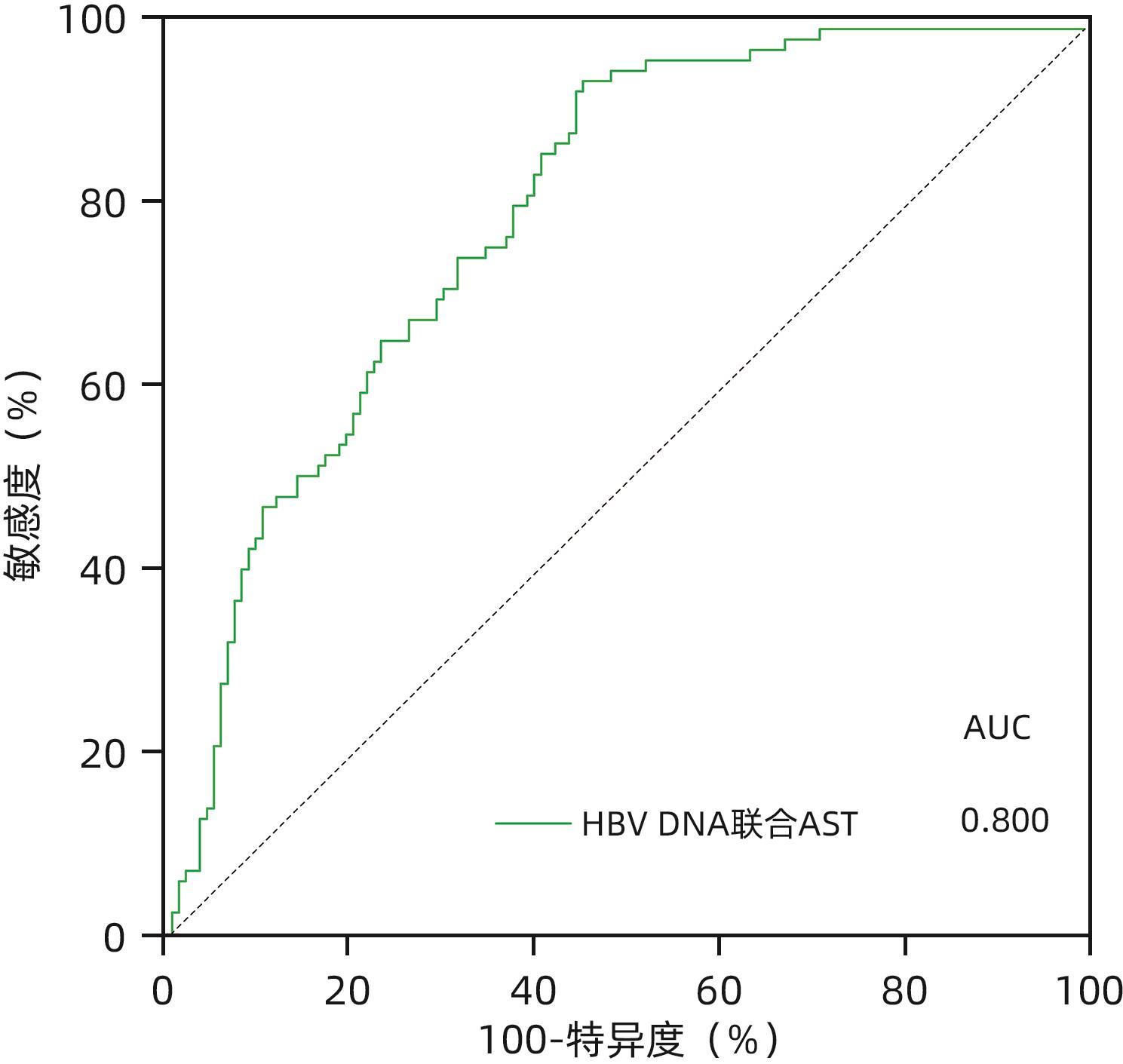
目的 慢性HBV感染自然史多有组织学意义上的免疫耐受状态,免疫耐受状态一旦打破,需立即启动抗病毒治疗。通过对免疫介导的肝损伤与HBV病毒学指标进行相关性分析,精准定义肝组织学免疫耐受状态的患者。 方法 以2010年1月—2022年12月中国人民解放军总医院第五医学中心、天津市第二人民医院、上海瑞金医院和浙江省台州医院收集的HBeAg阳性且HBV DNA>2×106 IU/mL未经抗病毒治疗的577例慢性乙型肝炎患者为研究对象。通过肝组织活检确定肝损伤情况,检测血清ALT、AST水平及病毒学指标。分析现有各大指南无创指标特别是HBV DNA载量界值不同的情况下,肝组织免疫耐受状态患者所占比例。进一步尝试以与肝组织免疫病理损伤程度相关性消失时的血清HBV DNA为新的阈值,联合多因素指标建立组织学免疫耐受状态的诊断模型。计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用Spearman法进行相关性分析。采用二元Logistic回归分析建立多因素诊断模型,并应用受试者工作特征曲线下面积(AUC)明确各模型的诊断效能,AUC的比较采用Z检验。 结果 中国2022版、EASL 2017版、AASLD 2018版、APASL 2015版慢性乙型肝炎防治指南病毒学无创指标所鉴定出的免疫耐受状态患者中符合本研究定义(HBV DNA>2×106 IU/mL)的组织学免疫耐受状态的比例分别为47.0%、38.5%、36.0%、44.6%,均未超过50%。当将血清HBV DNA阈值升至>2×108 IU/mL时,尽管免疫介导的肝损伤与HBV DNA相关性消失(r=-0.029,P=0.704),此时肝组织免疫耐受状态患者所占比例也仅为52.0%。在血清HBV DNA>1×108的HBeAg阳性患者队列(n=251)中,显著肝损伤组(n=140)和无显著肝损伤组(n=111)HBsAg、HBeAg、HBV DNA、ALT及AST水平比较,差异均有统计学意义(P值均<0.05),进一步多因素二元Logistic回归分析结果显示,AST、HBV DNA、HBeAg是患者组织学免疫耐受状态的独立影响因素(P值均<0.05)。根据以上指标并结合临床,建立预测模型:logit(P)=1.424-0.028×AST,AUC为0.730,AST最佳临界值为30.5 U/L,敏感度和特异度分别为52.8%、84.1%。进一步使用浙江省台州医院接受肝穿刺活检的238例成人慢性HBV感染者作为验证队列,验证结果显示,本研究建立的模型预测效能优于AST/ALT、FIB-4、APRI(AUC分别为0.698、0.555、0.518、0.373,P值均<0.05)。 结论 在HBV DNA>2×108 IU/mL的HBeAg阳性慢性HBV感染者中,AST>30.5 U/L时,提示组织学免疫耐受状态可能被“打破”。 Abstract:Objective The natural history of chronic HBV infection often involves a histologically defined immune tolerance state, and once such immune tolerance state is broken, antiviral therapy should be initiated immediately. This study aims to investigate the correlation between immune-mediated liver injury and virological indicators for HBV and precisely identify the patients with a histologically defined immune tolerance state. Methods This study was conducted among 577 HBeAg-positive chronic hepatitis B (CHB) patients with HBV DNA >2×106 IU/mL who did not receive antiviral therapy in The Fifth Medical Center of PLA General Hospital, Tianjin Second People’s Hospital, Shanghai Ruijin Hospital, and Taizhou Hospital of Zhejiang Province from January 2010 to December 2022. Liver biopsy was performed to determine the extent of liver injury, and the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and virological indicators were measured. The proportion of patients with a histologically defined immune tolerance state was analyzed based on the cut-off values of noninvasive indicators recommended in various guidelines, especially HBV load. In addition, a diagnostic model was established for the histologically defined immune tolerance state based on serum HBV DNA at the time when its correlation with liver immunopathological injury disappeared as the new threshold in combination with multiple indicators. The Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. The Spearman method was used for correlation analysis. The binary Logistic regression analysis was used to establish a multivariate diagnostic model; the area under the receiver operating characteristic curve (AUC) was used to investigate the diagnostic efficiency of different models, and the Z test was used for comparison of AUC. Results Among the patients with an immune tolerance state defined by the noninvasive indicators in the Chinese guidelines (2022 edition), the EASL guidelines (2017 edition), the AASLD guidelines (2018 edition), and the APASL guidelines (2015 edition) for the prevention and treatment of CHB, the patients with a histologically defined immune tolerance state who met the definition in this article (HBV DNA>2×106 IU/mL) accounted for 47.0%, 38.5%, 36.0%, and 44.6%, respectively, which did not exceed 50%. When the threshold of serum HBV DNA increased to >2×108 IU/mL, although the correlation between immune-mediated liver injury and HBV DNA disappeared (r=-0.029, P=0.704), the patients with a histologically defined immune tolerance state reached only 52.0%. In the cohort of 251 HBeAg-positive patients with serum HBV DNA >1×108 IU/mL, there were significant differences in the levels of HBsAg, HBeAg, HBV DNA, ALT, and AST between the significant liver injury group with 140 children and the non-significant liver injury group with 111 patients (all P<0.05), and the multivariate binary Logistic regression analysis showed that AST, HBV DNA, and HBeAg were influencing factors for histologically defined immune tolerance state in patients (all P<0.05). Based on the above indicators and related clinical data, a predictive model was established as logit(P)=1.424-0.028×AST, with an AUC of 0.730, an optimal cut-off value of 30.5 U/L, a sensitivity of 52.8%, and a specificity of 84.1%. A total of 238 adult patients with chronic HBV infection who underwent liver biopsy in Taizhou Hospital of Zhejiang Province were enrolled as the validation cohort, and the analysis showed that the predictive model established in this study had a better efficiency than AST/ALT, FIB-4, and APRI, with an AUC of 0.698, 0.555, 0.518, and 0.373, respectively (all P<0.05). Conclusion For HBeAg-positive patients with chronic HBV infection and HBV DNA>2×108 IU/mL, an AST level of >30.5 U/L might indicate the “breakdown” of histologically defined immune tolerance state. -
Key words:
- Hepatitis B, Chronic /
- Immune Tolerance /
- Diagnosis
-
表 1 入组患者临床特征
Table 1. Clinical characteristics of the enrolled patients
指标 总计(n=577) 存在显著肝损伤
(n=369)
无显著肝损伤
(n=208)
统计值 P值 年龄(岁) 33(27~39) 33(27~39) 33(27~40) Z=0.156 0.693 性别[例(%)] χ2=1.255 0.263 男 329(57.02) 204(55.30) 125(60.10) 女 248(42.98) 165(44.70) 83(39.90) ALT(U/L) 52.00(30.00~100.00) 66.00(38.00~142.30) 34.00(25.00~67.70) Z=34.830 <0.001 AST(U/L) 40.00(27.00~66.00) 46.00(32.10~97.00) 29.50(24.00~42.00) Z=38.366 <0.001 HBsAg(log10IU/mL) 4.25(3.76~4.67) 4.06(3.64~4.51) 4.60(4.31~4.84) Z=60.997 <0.001 HBeAg(log10S/CO) 3.09(2.59~3.19) 2.96(2.26~3.15) 3.17(3.09~3.23) Z=50.821 <0.001 HBV DNA(log10IU/mL) 7.84(7.32~8.40) 7.77(7.23~8.22) 8.16(7.58~8.62) Z=26.921 <0.001 表 2 各大CHB防治指南定义免疫耐受期的病毒学指标及诊断效能
Table 2. Virological indicators and diagnostic efficacy of the immunotolerant phase defined by the major guidelines for the prevention and treatment of chronic hepatitis B
表 3 不同HBV DNA截断值肝脏炎症活动度与病毒学指标之间的相关性
Table 3. Correlation between liver inflammatory activity and virological indicators at different HBV DNA cut-off values
变量 HBV DNA
>2×106 IU/mL
(n=577)[r(P)]
HBV DNA
>2×107 IU/mL
(n=438)[r(P)]
HBV DNA
>1×108 IU/mL
(n=251)[r(P)]
HBV DNA
>2×108 IU/mL(n=171)[r(P)]
HBV DNA 肝脏炎症活动度 -0.214(<0.001) -0.219(<0.001) -0.224(<0.001) -0.029(0.704) 肝脏纤维化分级 -0.243(<0.001) -0.122(0.011) -0,106(0.094) 0.054(0.704) ALT -0.049(0.236) -0.035(0.460) 0.044(0.484) 0.090(0.243) AST -0.119(0.004) -0.074(0.123) -0.003(0.968) 0.114(0.137) HBsAg 肝脏炎症活动度 -0.404(<0.001) -0.439(<0.001) -0.424(<0.001) -0.277(<0.001) 肝脏纤维化分级 -0.365(<0.001) -0.306(<0.001) -0.297(<0.001) -0.150(0.070) ALT -0.171(<0.001) -0.235(<0.001) -0.232(<0.001) -0.300(<0.001) AST -0.291(<0.001) -0.360(<0.001) -0.331(<0.001) -0.331(<0.001) HBeAg 肝脏炎症活动度 -0.405(<0.001) -0.445(<0.001) -0.403(<0.001) -0.348(<0.001) 肝脏纤维化分级 -0.411(<0.001) -0.378(<0.001) -0.309(<0.001) -0.219(<0.001) ALT -0.151(<0.001) -0.230(<0.001) -0.287(<0.001) -0.309(<0.001) AST -0.211(<0.001) -0.264(<0.001) -0.328(<0.001) -0.326(<0.001) 表 4 血清HBV DNA>1×108 IU/mL入组患者的临床特征
Table 4. Clinical characteristics of the enrolled patients with serum HBV DNA>1×108 IU/mL
指标 总计(n=251) 存在显著肝损伤
(n=140)
无显著肝损伤
(n=111)
统计值 P值 年龄(岁) 32(26~39) 31(26~38) 34(26~39) Z=-1.938 0.053 性别[例(%)] χ2=0.014 0.905 男 155(61.75) 86(61.43) 69(62.16) 女 96(38.25) 54(38.57) 42(37.84) ALT(U/L) 47.50(29.00~97.00) 66.00(37.00~163.00) 34.00(24.00~65.00) Z=-5.616 <0.001 AST(U/L) 37.00(25.00~61.25) 47.00(33.00~90.00) 27.00(22.00~39.00) Z=-6.536 <0.001 HBsAg(log10 IU/mL) 4.61(4.23~4.85) 4.41(4.07~4.75) 4.75(4.55~4.94) Z=-5.432 <0.001 HBeAg(log10 S/CO) 3.15(3.03~3.22) 3.11(2.96~3.18) 3.20(3.14~3.28) Z=-5.268 <0.001 HBV DNA(log10 IU/mL) 8.47(8.23~8.75) 8.35(8.17~8.71) 8.57(8.32~8.86) Z=-3.242 <0.001 表 5 血清HBV DNA>1×108 IU/mL患者的组织学免疫耐受状态多因素二元Logistic回归分析
Table 5. Multivariate binary Logistic regression analysis of histologically defined immune tolerance state in patients with serum HBV DNA>1×108 IU/mL
自变量 回归系数 Wald χ2 P值 OR 95%CI HBsAg (log10 IU/mL) 0.426 0.661 0.416 1.532 0.548~4.280 HBeAg(log10 S/Co) 1.667 4.564 0.033 5.297 1.144~24.526 HBV DNA(log10 IU/mL) 1.354 7.605 0.006 3.874 1.480~10.145 ALT(U/L) 0.003 0.163 0.687 1.003 0.990~1.016 AST(U/L) -0.054 9.693 0.002 0.948 0.916~0.980 -
[1] NGUYEN MH, WONG G, GANE E, et al. Hepatitis B virus: Advances in prevention, diagnosis, and therapy[J]. Clin Microbiol Rev, 2020, 33( 2): e00046-19. DOI: 10.1128/CMR.00046-19. [2] CHU CM, KARAYIANNIS P, FOWLER MJ, et al. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum[J]. Hepatology, 1985, 5( 3): 431- 434. DOI: 10.1002/hep.1840050315. [3] KENNEDY PTF, SANDALOVA E, JO J, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B[J]. Gastroenterology, 2012, 143( 3): 637- 645. DOI: 10.1053/j.gastro.2012.06.009. [4] MASON WS, GILL US, LITWIN S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant[J]. Gastroenterology, 2016, 151( 5): 986- 998. e 4. DOI: 10.1053/j.gastro.2016.07.012. [5] NING J, WANG JW, ZHENG HL, et al. Solely HBsAg intrauterine exposure accelerates HBV clearance by promoting HBs-specific immune response in the mouse pups[J]. Emerg Microbes Infect, 2022, 11( 1): 1356- 1370. DOI: 10.1080/22221751.2022.2071172. [6] WANG WT, ZHAO XQ, LI GP, et al. Immune response pattern varies with the natural history of chronic hepatitis B[J]. World J Gastroenterol, 2019, 25( 16): 1950- 1963. DOI: 10.3748/wjg.v25.i16.1950. [7] MILICH DR. The concept of immune tolerance in chronic hepatitis B virus infection is alive and well[J]. Gastroenterology, 2016, 151( 5): 801- 804. DOI: 10.1053/j.gastro.2016.09.037. [8] LIAW YF, CHU CM. Immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152( 5): 1245- 1246. DOI: 10.1053/j.gastro.2016.11.057. [9] XING TJ, ZHAO KY, LI WT, et al. Association between HBV viral load and severity of liver inflammation in patients with chronic hepatitis B virus infection[J]. Chin J Hepatol, 2023, 31( 9): 954- 960. DOI: 10.3760/cma.j.cn501113-20230820-00061.邢同京, 赵坤宇, 李文涛, 等. 慢性HBV感染者病毒DNA水平与患者肝组织炎症损伤程度的相关性研究[J]. 中华肝脏病杂志, 2023, 31( 9): 954- 960. DOI: 10.3760/cma.j.cn501113-20230820-00061. [10] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Inf, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [11] European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67( 2): 370- 398. DOI: 10.1016/j.jhep.2017.03.021. [12] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [13] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10( 1): 1- 98. DOI: 10.1007/s12072-015-9675-4. [14] ZHOU JL, WANG BQ, SUN YM, et al. Application value of liver stiffness measurement-to-platelet ratio index score in diagnosis of hepatitis B liver fibrosis and liver cirrhosis[J]. J Clin Hepatol, 2022, 38( 7): 1529- 1533. DOI: 10.3969/j.issn.1001-5256.2022.07.014.周家玲, 王冰琼, 孙亚朦, 等. LPRI评分在乙型肝炎肝纤维化及肝硬化中的诊断价值[J]. 临床肝胆病杂志, 2022, 38( 7): 1529- 1533. DOI: 10.3969/j.issn.1001-5256.2022.07.014. [15] LEE HW, CHAN HLY. Unresolved issues of immune tolerance in chronic hepatitis B[J]. J Gastroenterol, 2020, 55( 4): 383- 389. DOI: 10.1007/s00535-020-01665-z. -



 PDF下载 ( 921 KB)
PDF下载 ( 921 KB)

 下载:
下载: