Δ总胆红素-甲胎蛋白评分模型对HBV相关慢加急性肝衰竭短期预后的预测价值
DOI: 10.12449/JCH241209
Value of Δtotal bilirubin-alpha-fetoprotein scoring model in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure
-
摘要:
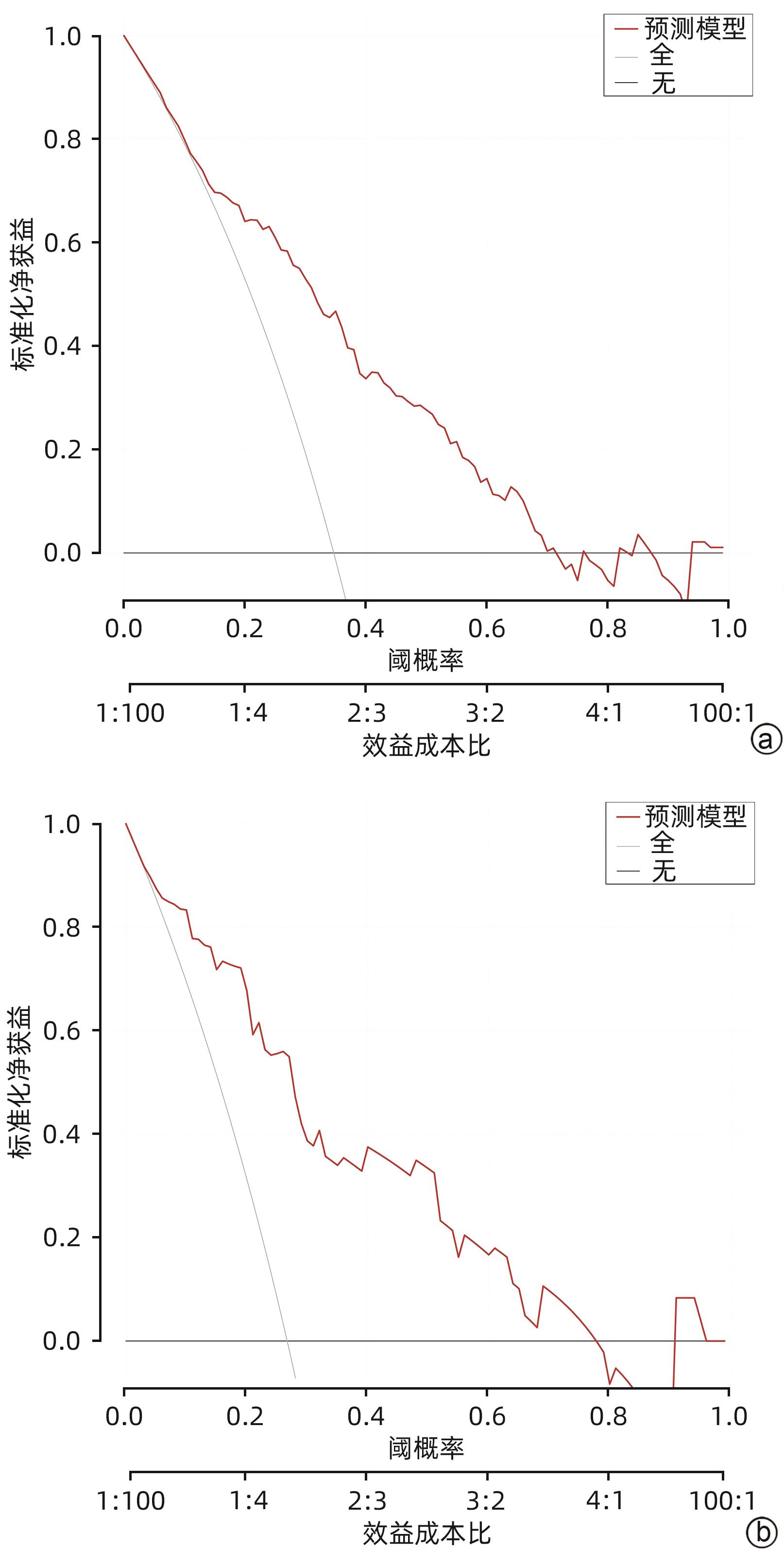
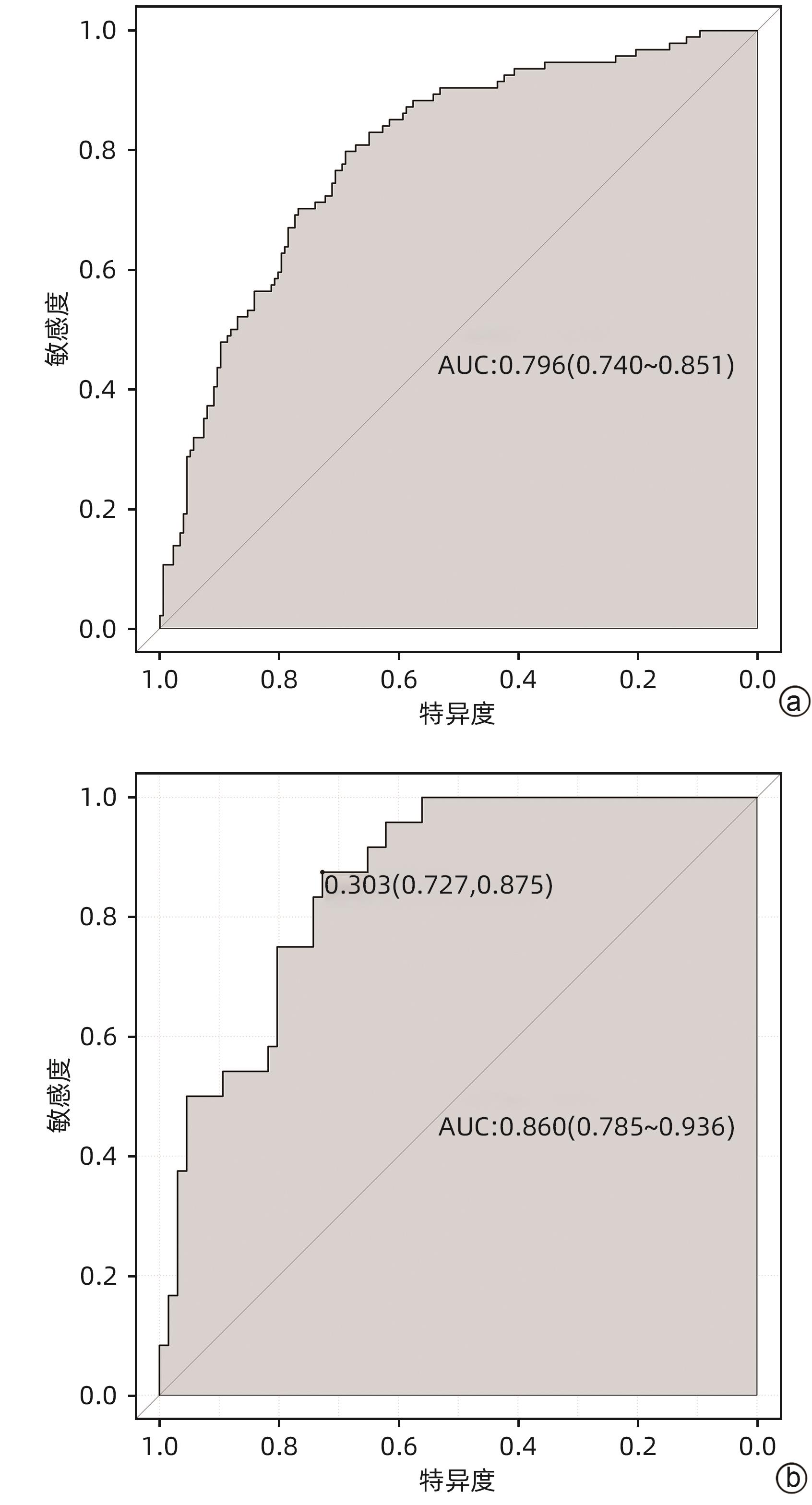
目的 探索血清总胆红素的动态变化(Δ总胆红素)及甲胎蛋白与HBV相关慢加急性肝衰竭(HBV-ACLF)患者短期预后的相关性,并建立新的评分模型,与MELD等评分模型对比分析新模型在HBV-ACLF短期预后中的作用价值。 方法 选取2015年1月—2022年12月于西部战区总医院消化内科住院治疗的HBV-ACLF患者作为回顾性研究队列。收集所有患者入院24 h临床资料,根据随访90天后的生存情况将患者分为生存组和死亡组。符合正态分布的计量资料组间比较采用成组t检验;偏态资料2组间比较采用Mann -Whitney U检验。计数资料2组间比较采用χ2检验或校正χ2检验。采用Logistic多因素回归分析,得出影响HBV-ACLF患者预后的危险因素并建立预后预测模型,采用受试者工作特征曲线(ROC曲线)评价新模型对于HBV-ACLF患者短期预后的预测价值。 结果 共纳入361例患者,90天生存率为67.3%(243/361),死亡组(n=118)年龄、上消化道出血发生率、肝性脑病发生率、INR、PT、白细胞、单核细胞、中性粒细胞、肌酐、Δ总胆红素、MELD评分、ALBI评分均高于生存组(n=243)(P值均<0.05);TC、HDL、LDL、白蛋白、甲胎蛋白、血小板、淋巴细胞、Na+均显著低于生存组(P值均<0.05)。多因素Logistic回归分析显示,AFP、PT、Na+、Δ总胆红素是HBV-ACLF患者90天预后的独立影响因素(P值均<0.05)。获得新的预测模型,即Δ总胆红素-甲胎蛋白评分模型:11.987+1.168×Δ总胆红素(%)-0.095×Na+(mmol/L)+ 0.25×PT(s)-0.002×AFP(ng/mL),由此构建的模型具有较高预测价值(AUC为0.796,敏感度0.766,特异度0.723),决策曲线提示获益较好。 结论 Δ总胆红素-甲胎蛋白评分模型与MELD评分、ALBI等常用预测模型相比,具有较高预测效能。 Abstract:Objective To investigate the association of the dynamic changes of serum total bilirubin (ΔTBil) and alpha-fetoprotein (AFP) with the short-term prognosis of patients with hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF), to establish a new scoring model, and to investigate the value of this model in evaluating the short-term prognosis of HBV-ACLF through comparison with Model for End-Stage Liver Disease (MELD) score and other scoring systems. Methods The patients with HBV-ACLF who were hospitalized and treated in Department of Gastroenterology, The General Hospital of Western Theater Command, from January 2015 to December 2022 were enrolled as the retrospective study cohort. Clinical data within 24 hours after admission were collected from all patients, and the patients were divided into survival group and death group according to the survival after 90 days of follow-up. The independent-samples t test was used for comparison of normally distributed continuous data between groups; the Mann-Whitney U test was used for comparison of continuous data with skewed distribution between groups; the chi-square test or the corrected chi-square test was used for comparison of categorical data between two groups. A multivariate Logistic regression analysis was used to determine the risk factors for the prognosis of HBV-ACLF patients and establish a predictive model for prognosis, and the receiver operating characteristic (ROC) curve was used to investigate the value of the new model in predicting the short-term prognosis of HBV-ACLF patients. Results A total of 361 patients were included in the analysis, with a 90-day survival rate of 67.3% (243/361). Compared with the survival group (n=243), the death group (n=118) had significantly higher age, incidence rates of upper gastrointestinal bleeding and hepatic encephalopathy, international normalized ratio, prothrombin time (PT), leukocytes, monocytes, neutrophils, creatinine, ΔTBil, MELD score, and ALBI score (all P<0.05), as well as significantly lower levels of total cholesterol, high-density lipoprotein, low-density lipoprotein, albumin, AFP, platelet count, lymphocytes, and Na+ (all P<0.05). The multivariate Logistic regression analysis showed that AFP, PT, Na+, and ΔTBil were independent influencing factors for the 90-day prognosis of patients with HBV-ACLF (all P<0.05). The new ΔTBil-AFP scoring model was established as 11.987+1.168×ΔTBil (%)-0.095×Na+ (mmol/L)+0.25×PT (s)-0.002×AFP (ng/mL), which had a relatively high predictive value, with an area under the ROC curve of 0.796, a sensitivity of 0.766, and a specificity of 0.723, and the decision curve showed good benefits. Conclusion Compared with the commonly used prediction models such as MELD score and ALBI score, the ΔTBil-AFP scoring model has a better prediction performance. -
Key words:
- Acute-On-Chronic Liver Failure /
- Hepatitis B Virus /
- Bilirubin /
- alpha-Fetoproteins /
- Prognosis
-
表 表1 生存组和死亡组HBV-ACLF患者临床指标比较
Table 表1. Comparison of various indicators between survival and non-survival group
项目 生存组(n=243) 死亡组(n=118) 统计值 P值 男[例(%)] 202.0(83.1) 99.0(83.9) χ2=0.034 >0.05 年龄(岁) 48.23±12.23 54.42±11.05 t=-4.821 <0.001 上消化道出血[例(%)] 7.0(2.9) 20.0(16.9) χ2=22.719 <0.001 肝性脑病[例(%)] 17.0(7.0) 54.0(45.8) χ2=75.554 <0.001 TC(mmol/L) 2.21(1.72~2.81) 1.85(1.34~2.23) Z=-5.108 <0.001 HDL(mmol/L) 0.36(0.24~0.59) 0.28(0.16~0.43) Z=-3.607 <0.001 LDL(mmol/L) 1.58(1.26~2.03) 1.34(1.01~1.74) Z=-4.099 <0.001 ALT(U/L) 457.0(168.5~1 062.2) 350.5(140.7~804.8) Z=-1.639 0.101 AST(U/L) 366.7(149.0~766.3) 314.9(141.8~890.1) Z=-0.357 0.721 白蛋白(g/L) 31.8(28.8~34.4) 31.0(27.7~33.4) Z=-2.409 0.016 AFP(ng/mL) 83.50(21.68~228.81) 29.29(10.92~85.14) Z=-4.662 <0.001 INR 1.68(1.52~1.91) 2.01(1.74~2.41) Z=-6.707 <0.001 PT(s) 18.9(17.3~21.6) 22.9(19.5~27.3) Z=-7.053 <0.001 白细胞(×109) 5.65(4.38~7.50) 6.76(5.08~8.54) Z=-3.299 <0.001 血小板(×109) 100.0(58.0~127.0) 79.5(50.0~101.2) Z=-3.799 <0.001 单核细胞(×109/L) 0.49(0.35~0.68) 0.61(0.42~0.86) Z=-3.818 <0.001 淋巴细胞(×109/L) 1.01(0.73~1.45) 0.82(0.55~1.08) Z=-3.858 <0.001 中性粒细胞(×109/L) 3.99(2.77~5.41) 5.00(3.57~6.83) Z=-4.026 <0.001 肌酐(μmol/L) 73.0(62.0~86.0) 77.5(64.8~98.1) Z=-2.354 0.019 Na+(mmol/L) 136.3(133.9~138.4) 134.4(131.7~136.4) Z=-4.903 <0.001 K+(mmol/L) 3.87(3.58~4.20) 3.82(3.48~4.15) Z=-0.910 0.363 Δ总胆红素(%) -0.210(-0.420~0.004) 0.040(-0.140~0.300) Z=-6.414 <0.001 MELD评分(分) 21.13(18.68~23.53) 24.38(21.57~28.32) Z=-7.502 <0.001 ALBI评分(分) -3.63(-3.90~-3.27) -3.24(-3.30~-2.96) Z=-6.288 <0.001 表 2 训练集与验证集HBV-ACLF患者临床特征比较
Table 2. Comparison of clinical features of HBV-ACLF patients in training and verification
项目 训练集(n=271) 验证集(n=90) 统计值 P值 年龄(岁) 50.24±12.07 50.29±12.62 t=-0.030 0.976 上消化道出血[例(%)] 17.00(6.30) 10.00(11.10) χ2=2.285 0.163 肝性脑病[例(%)] 55.00(20.30) 16.00(17.80) χ2=0.271 0.649 TC(mmol/L) 2.03(1.60~2.63) 1.96(1.49~2.66) Z=-0.342 0.734 HDL(mmol/L) 0.34(0.22~0.55) 0.32(0.19~0.47) Z=-0.848 0.396 LDL(mmol/L) 1.54(1.20~1.95) 1.51(1.16~1.83) Z=-0.880 0.379 ALT(U/L) 415.30(140.70~1 024.80) 467.20(187.50~925.60) Z=-0.570 0.569 AST(U/L) 308.80(143.80~801.40) 380.70(163.70~758.80) Z=-0.809 0.418 白蛋白(g/L) 31.50(28.30~34.00) 31.90(28.80~34.20) Z=-0.674 0.500 AFP(ng/mL) 59.70(16.70~165.30) 67.87(18.90~233.80) Z=-1.078 0.281 INR 1.78(1.57~2.09) 1.75(1.58~2.11) Z=-0.015 0.988 PT(s) 20.00(17.70~23.50) 19.41(17.74~23.95) Z=-0.326 0.745 白细胞(×109) 5.94(4.55~7.87) 6.12(4.09~8.04) Z=-0.421 0.674 血小板(×109) 90.00(61.00~119.00) 94.50(62.75~128.00) Z=-0.867 0.386 单核细胞(×109/L) 0.54(0.38~0.72) 0.50(0.35~0.76) Z=-0.364 0.716 淋巴细胞(×109/L) 0.95(0.72~1.33) 0.87(0.58~1.42) Z=-1.271 0.204 中性粒细胞(×109/L) 4.19(2.95~5.79) 4.42(2.86~5.83) Z=-0.266 0.790 肌酐(μmol/L) 74.00(64.00~89.00) 73.00(62.00~88.00) Z=-0.440 0.660 Na+(mmol/L) 135.60(133.20~138.20) 135.30(132.20~138.10) Z=-0.909 0.363 K+(mmol/L) 3.87(3.54~4.14) 3.88(3.57~4.25) Z=-0.807 0.420 Δ总胆红素(%) -0.10(-0.32~0.16) -0.17(-0.37~0.01) Z=-2.142 0.320 MELD评分(分) 22.01(19.13~25.07) 22.12(19.26~25.02) Z=-0.041 0.967 ALBI评分(分) -3.47(-3.80~-3.09) -3.52(-3.81~-3.18) Z=-0.559 0.576 表 3 HBV-ACLF患者90天预后Logistic回归分析
Table 3. Independent factors for the 90-day prognosis of patients with HBV-ACLF
参数 单因素分析 多因素分析 β值 OR(95%CI) P值 β值 OR(95%CI) P值 TC -0.650 0.522(0.370~0.737) <0.001 HDL -1.156 0.209(0.065~0.670) 0.008 白蛋白 -0.067 0.936(0.886~0.988) 0.017 INR 1.544 4.685(2.465~8.904) <0.001 PT 0.149 1.161(1.094~1.232) <0.001 0.250 1.284(1.011~1.630) 0.040 AFP -0.002 0.998(0.996~0.999) 0.006 -0.002 0.998(0.997~1.000) 0.016 血小板 -0.007 0.993(0.987~0.999) 0.025 Na+ -0.104 0.901(0.850~0.955) <0.001 -0.095 0.909(0.850~0.972) 0.005 Δ总胆红素 1.013 2.755(1.569~4.837) <0.001 1.168 3.215(1.575~6.562) 0.001 表 4 Δ总胆红素-甲胎蛋白评分模型与MELD、MELD-Na、ALBI评分模型比较
Table 4. Comparison ROC between Δ total bilirubin-alpha-fetoprotein scoring model and MELD, MELD-Na, ALBI scoring model
评分模型 AUC cut-off值 约登指数 敏感度 特异度 Δ总胆红素-甲胎蛋白评分模型 0.796 3.955 0.489 0.766 0.723 MELD评分 0.708 23.870 0.344 0.564 0.780 MELD-Na评分 0.707 22.430 0.370 0.681 0.689 ALBI评分 0.707 -3.475 0.355 0.734 0.621 -
[1] CHEN J, SU HB, HU JH. An excerpt of acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver(APASL) 2019 update[J]. J Clin Hepatol, 2019, 35( 9): 1933- 1936. DOI: 10.3969/j.issn.1001-5256.2019.09.009.陈婧, 苏海滨, 胡瑾华.《2019年亚太肝病学会共识建议: 慢加急性肝衰竭管理更新》摘译[J]. 临床肝胆病杂志, 2019, 35( 9): 1933- 1936. DOI: 10.3969/j.issn.1001-5256.2019.09.009. [2] CHEN X, ZHANG SY, ZHOU J, et al. The effect of diabetes mellitus on the post-transplant survival rate of liver transplant recipients with different grades of acute-on-chronic liver failure[J/CD]. Chin J Transplant(Electronic Edition), 2022, 16( 2): 72- 77. DOI: 10.3877/cma.j.issn.1674-3903.2022.02.002.陈霞, 张思遥, 周杰, 等. 糖尿病对不同分级慢加急性肝衰竭肝移植受者术后生存率的影响[J/CD]. 中华移植杂志(电子版), 2022, 16( 2): 72- 77. DOI: 10.3877/cma.j.issn.1674-3903.2022.02.002. [3] HERNAEZ R, LIU Y, KRAMER JR, et al. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure[J]. J Hepatol, 2020, 73( 6): 1425- 1433. DOI: 10.1016/j.jhep.2020.06.005. [4] XUE H, MING F, ZHANG Y, et al. The prognostic value of ALBI score in patients with HBV related acute-on-chronic liver failure[J]. Med J Commun, 2022, 36( 1): 33- 36, 40. DOI: 10.19767/j.cnki.32-1412.2022.01.008.薛红, 明芳, 章颖, 等. ALBI评分对HBV相关慢加急性肝衰竭患者预后的预测价值[J]. 交通医学, 2022, 36( 1): 33- 36, 40. DOI: 10.19767/j.cnki.32-1412.2022.01.008. [5] SARIN SK, CHOUDHURY A. Acute-on-chronic liver failure: Terminology, mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2016, 13( 3): 131- 149. DOI: 10.1038/nrgastro.2015.219. [6] WANG ZC, SHAO JG, GU EL. Short term prediction of rebound rate of total bilirubin in patients with chronic suba-cute liver failure treated with artificial liver[J]. Chin J Infect Dis, 2013, 31( 11): 678- 680. DOI: 10.3760/cma.j.issn.1000-6680.2013.11.009.王忠成, 邵建国, 顾尔莉. 总胆红素反弹率对人工肝治疗慢加亚急性肝功能衰竭的短期预测[J]. 中华传染病杂志, 2013, 31( 11): 678- 680. DOI: 10.3760/cma.j.issn.1000-6680.2013.11.009. [7] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Disease, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [8] GUO BC, LI YH, CHEN R, et al. Value of MELD 3.0, MELD, and MELD-Na scores in assessing the short-term prognosis of patients with acute-on-chronic liver failure: A comparative study[J]. J Clin Hepatol, 2023, 39( 11): 2635- 2642. DOI: 10.3969/j.issn.1001-5256.2023.11.018.郭北辰, 李雨韩, 陈蕊, 等. MELD 3.0、MELD和MELD-Na评分对慢加急性肝衰竭患者短期预后的评估价值[J]. 临床肝胆病杂志, 2023, 39( 11): 2635- 2642. DOI: 10.3969/j.issn.1001-5256.2023.11.018. [9] LI YY, XU L. Relationship between serum prealbumin and total bilirubin ratio, ALBI score, MELD score and prognosis of acute-on-chronic liver failure[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30( 5): 417- 419. DOI: 10.3969/j.issn.1005-0264.2020.05.011.李艳艳, 徐龙. 血清前白蛋白与总胆红素比值、ALBI评分、MELD评分与慢加急性肝衰竭患者预后的关系[J]. 中西医结合肝病杂志, 2020, 30( 5): 417- 419. DOI: 10.3969/j.issn.1005-0264.2020.05.011. [10] FAN Q, LI Z. Liver transplantation for acute-on-chronic liver failure[J]. Ogran Transplant, 2022, 13( 3): 333- 337. DOI: 10.3969/j.issn.1674-7445.2022.03.008.范祺, 李照. 慢加急性肝衰竭的肝移植治疗[J]. 器官移植, 2022, 13( 3): 333- 337. DOI: 10.3969/j.issn.1674-7445.2022.03.008. [11] GAO X, ZHAO CJ, HU SH. Short-term prognostic value of age-bilirubin-international normalized ratio-creatinine score in patients with hepatitis B virus-related acute-on-chronic liver failure[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2022, 16( 2): 108- 114. DOI: 10.3877/cma.j.issn.1674-1358.2022.02.005.高祥, 赵成军, 胡世宏. 年龄-胆红素-国际标准化比率-肌酐评分对乙型肝炎相关慢加急性肝功能衰竭患者短期预后的评估价值[J/CD]. 中华实验和临床感染病杂志(电子版), 2022, 16( 2): 108- 114. DOI: 10.3877/cma.j.issn.1674-1358.2022.02.005. [12] YU ZJ, ZHANG Y, CAO YY, et al. A dynamic prediction model for prognosis of acute-on-chronic liver failure based on the trend of clinical indicators[J]. Sci Rep, 2021, 11( 1): 1810. DOI: 10.1038/s41598-021-81431-0. [13] GUSTOT T, FERNANDEZ J, GARCIA E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis[J]. Hepatology, 2015, 62( 1): 243- 252. DOI: 10.1002/hep.27849. [14] PEREIRA G, BALDIN C, PIEDADE J, et al. Combination and sequential evaluation of acute-on-chronic liver failure(ACLF) and hyponatremia and prognosis in cirrhotic patients[J]. Dig Liver Dis, 2020, 52( 1): 91- 97. DOI: 10.1016/j.dld.2019.08.013. [15] CHEN W. Predictive value of end-stage liver disease model combined with serum sodium score in short-term prognosis of patients with liver failure[J]. J N Sichuan Med Coll, 2024, 39( 2): 247- 251.陈炜. 终末期肝病模型联合血清钠评分对肝衰竭患者短期预后的预测价值[J]. 川北医学院学报, 2024, 39( 2): 247- 251. [16] YANG B, WU YK, CHEN ZC, et al. The dynamic changes of AST, ALT, TBil and PT and their relationship with prognosis of acute-on-chronic hepatitis B liver failure[J]. J Clin Hepatol, 2012, 28( 3): 205- 208. DOI: 10.3969/j.issn.1001-5256.2012.03.012.杨波, 吴元凯, 陈忠诚, 等. ALT、AST、TBil及PT变化趋势与慢加急性乙型肝炎肝衰竭预后的关系[J]. 临床肝胆病杂志, 2012, 28( 3): 205- 208. DOI: 10.3969/j.issn.1001-5256.2012.03.012. [17] XIN KF, LI M, LI SS, et al. The role of TBARR, TBRR and TBCR in prognosis evaluation of patients with acute liver failure after plasma exchange therapy[J]. Shandong Med J, 2018, 58( 25): 44- 46. DOI: 10.3969/j.issn.1002-266X.2018.25.012.辛克锋, 李铭, 李莎莎, 等. TBARR、TBRR、TBCR在血浆置换治疗后慢加急性肝衰竭患者预后评估中的作用[J]. 山东医药, 2018, 58( 25): 44- 46. DOI: 10.3969/j.issn.1002-266X.2018.25.012. [18] TOMARO ML, BATLLE AM. Bilirubin: Its role in cytoprotection against oxidative stress[J]. Int J Biochem Cell Biol, 2002, 34( 3): 216- 220. DOI: 10.1016/s1357-2725(01)00130-3. [19] LONGHI MS, VUERICH M, KALBASI A, et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39[J]. JCI Insight, 2017, 2( 9): e92791. DOI: 10.1172/jci.insight.92791. [20] BERGSTRAND CG, CZAR B. Demonstration of a new protein fraction in serum from the human fetus[J]. Scand J Clin Lab Investig, 1956, 8( 2): 174. DOI: 10.3109/00365515609049266. [21] WANG XP, SHEN CF, YANG JJ, et al. Alpha-fetoprotein as a predictive marker for patients with hepatitis B-related acute-on-chronic liver failure[J]. Can J Gastroenterol Hepatol, 2018, 2018: 1232785. DOI: 10.1155/2018/1232785. [22] LI JN, HAN C, WANG XP, et al. Current research on liver regeneration: Pathogenesis and serum markers[J]. J Clin Hepatol, 2020, 36( 8): 1896- 1899. DOI: 10.3969/j.issn.1001-5256.2020.08.048.李嘉妮, 韩川, 王孝平, 等. 肝再生的发生机制及血清标志物的研究现状[J]. 临床肝胆病杂志, 2020, 36( 8): 1896- 1899. DOI: 10.3969/j.issn.1001-5256.2020.08.048. [23] WU H, LI H, TANG SH. Role of alpha-fetoprotein in prognostic evaluation of patients with liver failure[J]. J Clin Hepatol, 2021, 37( 11): 2706- 2709. DOI: 10.3969/j.issn.1001-5256.2021.11.048.吴红, 李浩, 汤善宏. 甲胎蛋白在肝衰竭患者预后判断中的作用[J]. 临床肝胆病杂志, 2021, 37( 11): 2706- 2709. DOI: 10.3969/j.issn.1001-5256.2021.11.048. [24] DONG JL, CHEN Y. Recognition of the clinical classification of acute-on-chronic liver failure: Redefinition from a new perspective of onset manifestations and dynamic outcomes[J]. J Clin Hepatol, 2023, 39( 10): 2277- 2280. DOI: 10.3969/j.issn.1001-5256.2023.10.002.董金玲, 陈煜. 慢加急性肝衰竭临床分型的再认识: 从起病表现和动态转归的新视角重新定义[J]. 临床肝胆病杂志, 2023, 39( 10): 2277- 2280. DOI: 10.3969/j.issn.1001-5256.2023.10.002. -



 PDF下载 ( 1077 KB)
PDF下载 ( 1077 KB)

 下载:
下载: