2018—2023年中药复方治疗非酒精性脂肪性肝炎的随机对照试验质量评价
DOI: 10.12449/JCH241210
Quality assessment of randomized controlled trials of compound traditional Chinese medicine prescriptions in treatment of nonalcoholic steatohepatitis in 2018—2023
-
摘要:
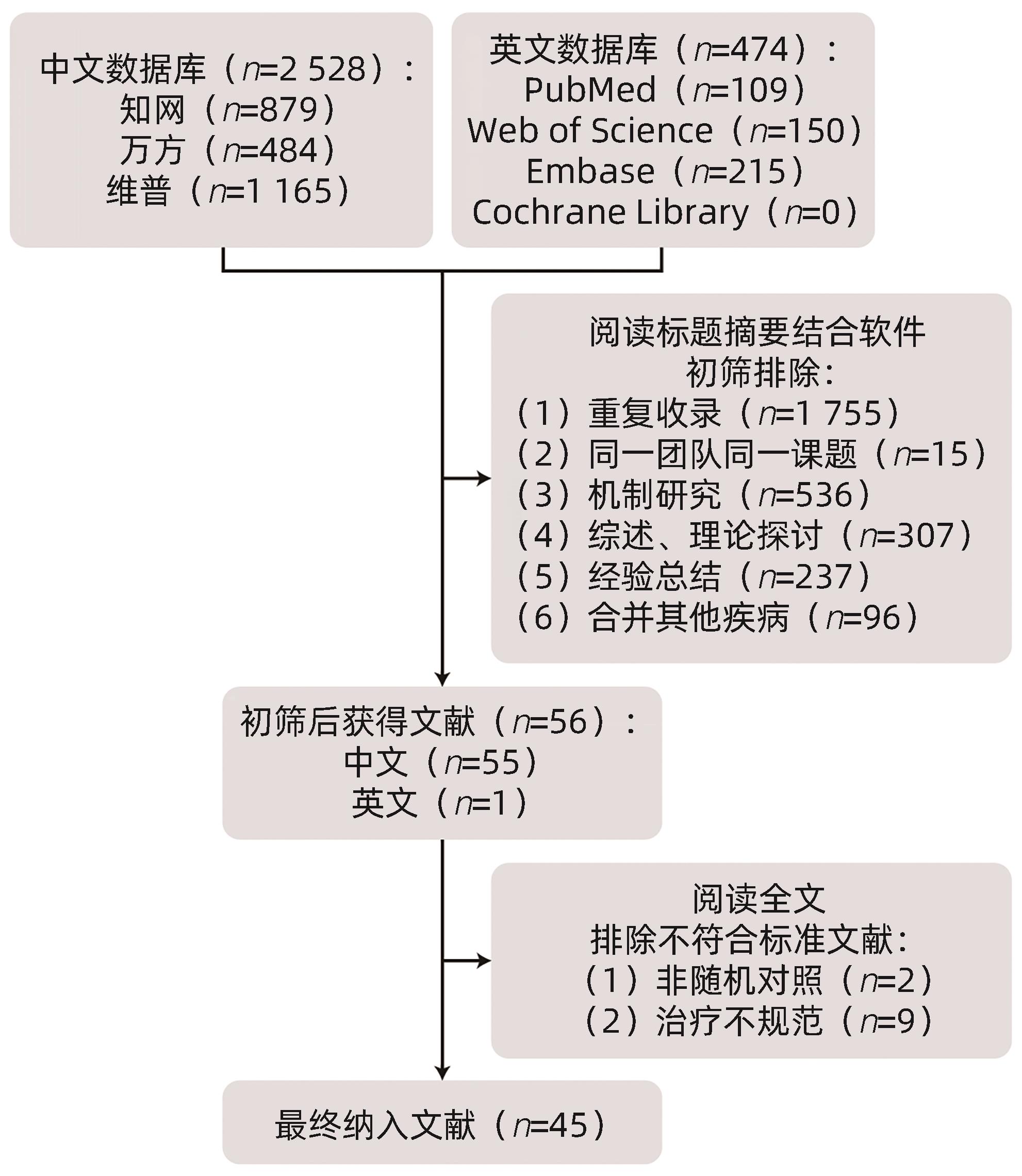
目的 评价中药复方治疗非酒精性脂肪性肝炎(NASH)随机对照试验(RCT)的研究质量,为规范该领域RCT的研究设计和报告提出建议。 方法 系统检索2018年1月1日—2023年12月31日PubMed、Web of Science、Embase、Cochrane Library、中国知网、维普和万方等数据库发表的中药复方干预NASH的RCT相关文献,参照Cochrane偏倚风险评估工具(RoB 2.0)和临床试验报告统一标准CONSORT 2010及针对中药复方CONSORT-CHM Formulas 2017扩展版分别对文献进行筛选评价及分析讨论。 结果 本研究最终纳入文献45篇,大部分研究被RoB 2.0评为高风险偏倚。根据CONSORT对照检查清单发现大部分关于RCT研究质量的关键条目报告率较低。 结论 近6年中药复方治疗NASH的临床研究偏倚风险较大,报告质量较差,从而可能造成证据质量不高,提示在挖掘中医药优势的同时更应注重临床研究的顶层设计,提高研究质量。 -
关键词:
- 非酒精性脂肪性肝病 /
- 中草药 /
- 复方 /
- 随机对照试验(主题)
Abstract:Objective To assess the quality of randomized controlled trials (RCTs) of compound traditional Chinese medicine (TCM) prescriptions in the treatment of nonalcoholic steatohepatitis (NASH), and to provide recommendations for standardizing the design and reporting of RCTs in this field. Methods Databases such as PubMed, Web of Science, Embase, the Cochrane Library, CNKI, VIP, and Wanfang Data were searched for RCTs of compound TCM prescriptions in the treatment of NASH published from January 1, 2018 to December 31, 2023, and the articles were screened and assessed based on the Cochrane risk-of-bias assessment tool (RoB 2), the unified standard for clinical trial reporting (CONSORT 2010), and CONSORT-CHM Formulas 2017 for compound TCM prescriptions. Results A total of 45 articles were finally included, and most of these studies were rated as high-risk bias by RoB 2.0. The analysis based on the CONSORT control checklist showed a relatively low reporting rate for most of the key items regarding the quality of RCT studies. Conclusion A relatively large risk of bias is observed in the clinical studies on compound TCM prescriptions in the treatment of NASH published in the past six years, which may lead to the poor quality of reporting and evidence. It is suggested that the top-level design of clinical studies should be taken seriously in addition to investigating the advantages of TCM, so as to improve the quality of clinical studies. -
表 1 纳入文献的基本特征
Table 1. Basic characteristics of the included literature
项目 文献篇数(%) 发表时间 2018年 15(33.33) 2019年 6(13.33) 2020年 9(20.00) 2021年 10(22.22) 2022年 3(6.67) 2023年 2(4.44) 样本量 ≥30~60例 35(77.78) ≥60~90例 9(20.00) ≥90例 1(2.22) 干预措施 中药汤剂 32(71.11) 中药颗粒 11(24.44) 中药胶囊 2(4.44) 疗程 <3个月 5(11.11) 3~6个月 32(71.11) ≥6个月 8(17.78) 表 2 基于CONSORT 2010原条目评价纳入RCT报告质量结果
Table 2. RCT reported quality results was evaluated based on the original CONSORT 2010 checklist
论文部分 条目 条目内容 报告篇数(%) 标题和摘要 1a 通过文题能够识别是随机临床试验 1(2.22) 1b 结构式摘要,包括试验设计、方法、结果、结论4个部分 45(100.00) 前言 背景 2a 科学背景和对试验理由的解释 43(95.56) 目的 2b 具体目的或假设 44(97.78) 方法 试验方案 3a 描述试验设计(诸如平行设计、析因设计),包括受试者分配至各组的比例 1(2.22) 3b 试验开始后对试验方法所作的重要改变(如合格受试者的挑选标准),并说明原因 0(0.00) 受试者 4a 受试者的合格标准 45(100.00) 4b 资料收集的场所和地点 45(100.00) 干预措施 5 详细描述各组干预措施的细节以使他人能够重复,包括实际上是在何时、如何实施的 45(100.00) 结局指标 6a 完整而确切地说明预先设定的主要和次要结局指标,包括是何时、如何测评的 45(100.00) 6b 试验开始后对结局指标是否有任何更改,并说明原因 0(0.00) 样本量 7a 如何确定样本量 0(0.00) 7b 必要时,解释中期分析和试验中止原则 0(0.00) 序列产生 8a 产生随机分配序列的方法 31(68.89) 8b 随机方法的类型,任何限定的细节(如何分区组和各区组样本量多少) 2(4.44) 分配隐藏机制 9 用于执行随机分配序列的机制(如按顺序编码的封藏法),描述干预措施分配之前为隐藏序列号所采取的步骤 1(2.22) 实施者 10 明确试验各步骤实施者身份 1(2.22) 盲法 11a 若实施了盲法,描述分配干预措施后对谁设盲(如受试者、干预实施者、结局评估者),以及盲法是如何实施的 1(2.22) 11b 如有必要,描述干预措施的相似之处 0(0.00) 统计学方法 12a 用于比较各组主要和次要结局指标的统计学方法 45(100.00) 12b 附加的分析方法,诸如亚组分析和校正分析 0(0.00) 结果 受试者流程 13a 随机分配到各组的受试者例数,接受已知分配治疗的例数,纳入主要结局分析的例数 45(100.00) 13b 随机分组后,各组脱落和被剔除的例数,并说明原因 5(11.11) 招募受试者 14a 招募期和随访时间的长短,并说明具体日期 0(0.00) 14b 试验中断或停止的原因 0(0.00) 基线资料 15 用表格呈现每组受试者的基线数据,包括人口学资料和临床特征 0(0.00) 纳入例数 16 描述各组纳入分析的受试者数目(分母),以及是否按最初的分组进行分析 45(100.00) 结果和估计值 17a 各组每一项主要和次要结局指标的结果,效应估计值,及其精确性(如95%可信区间) 45(100.00) 17b 对于二分类结局,建议同时提供相对效应值和绝对效应值 0(0.00) 辅助分析 18 各组出现的所有严重危害或意外效应 0(0.00) 危害 19 各组产生的不良反应,或意外效应 7(15.56) 讨论 局限性 20 试验的局限性,报告潜在偏倚和不精确的原因,以及出现多种分析结果的原因 0(0.00) 可推广性 21 试验结果被推广的可能性(外部可靠性,实用性) 17(37.78) 解释 22 与结果相对应的解释,权衡试验结果的利弊,并且考虑其他相关证据 0(0.00) 其他信息 试验注册 23 临床试验注册号和注册机构名称 0(0.00) 试验方案 24 可能的话,告知从何处获取完整的试验方案 0(0.00) 资助 25 资助和其他支持(如提供药品)的来源,提供资助者所起的作用 24(53.33) 表 3 基于CONSORT-CHM Formulas 2017扩展版评价纳入RCT报告质量结果
Table 3. The RCT reporting quality results were included based on the CONSORT-CHM Formulas 2017 extension version evaluation
论文部分 条目 条目内容 报告篇数(%) 标题和摘要 1a 说明中药临床试验是针对某个中医证型、某个西医定义的疾病或某个具有特定中医证型的西医定义的疾病(如适用) 45(100.00) 1b 说明复方的名称、剂型及所针对的中医证型(如适用) 20(44.44) 1c 确定适当的关键词,包括“中药复方”和“随机对照试验” 21(46.67) 前言 背景 2a 基于生物医学理论和/或传统中医学理论的解释 36(80.00) 目的 2b 说明中药临床试验是针对某个中医证型、某个西医定义的疾病或某个具有特定中医证型的西医定义的疾病(如适用) 14(31.11) 方法 受试者 4a 若招募特定中医证型的受试者,应详细说明其诊断标准、纳入和排除标准。需使用公认的诊断标准,或提供参考出处,使读者能够详细查阅解释 41(91.11) 干预措施 5 不同类型的中药复方,包括不同的报告细节 0(0.00) 结局指标 6a 详细报告与中医证候相关的结局指标 34(75.56) 讨论 可推广性 21 讨论中药复方对不同中医证候和疾病的作用 4(8.89) 解释 22 以传统中医学理论作解释 44(97.78) -
[1] YOUNOSSI Z, ANSTEE QM, MARIETTI M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15( 1): 11- 20. DOI: 10.1038/nrgastro.2017.109. [2] RINELLA ME, LAZARUS JV, RATZIU V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature[J]. Hepatology, 2023, 78( 6): 1966- 1986. DOI: 10.1097/HEP.0000000000000520. [3] SONG SJ, LAI JC, WONG GL, et al. Can we use old NAFLD data under the new MASLD definition?[J]. J Hepatol, 2024, 80( 2): e54- e56. DOI: 10.1016/j.jhep.2023.07.021. [4] HARRISON SA, ALLEN AM, DUBOURG J, et al. Challenges and opportunities in NASH drug development[J]. Nat Med, 2023, 29( 3): 562- 573. DOI: 10.1038/s41591-023-02242-6. [5] Branch of Hepatobiliary Diseases, China Association of Chinese Medicine. Diagnosis and treatment guideline for Chinese medicine on non-alcoholic steatohepatitis[J]. J Clin Hepatol, 2023, 39( 5): 1041- 1048. DOI: 10.3969/j.issn.1001-5256.2023.05.007.中华中医药学会肝胆病分会. 非酒精性脂肪性肝炎中医诊疗指南[J]. 临床肝胆病杂志, 2023, 39( 5): 1041- 1048. DOI: 10.3969/j.issn.1001-5256.2023.05.007. [6] MISCIAGNA G, DEL PILAR DÍAZ M, CARAMIA DV, et al. Effect of a low glycemic index mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinici trial[J]. J Nutr Health Aging, 2017, 21( 4): 404- 412. DOI: 10.1007/s12603-016-0809-8. [7] HIGGINS JP, ALTMAN DG, GØTZSCHE PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials[J]. BMJ, 2011, 343: d5928. DOI: 10.1136/bmj.d5928. [8] SCHULZ KF, ALTMAN DG, MOHER D, et al. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials[J]. BMJ, 2010, 340: c332. DOI: 10.1136/bmj.c332. [9] CHENG CW, WU TX, SHANG HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: Recommendations, explanation, and elaboration[J]. Ann Intern Med, 2017, 167( 2): 112- 121. DOI: 10.7326/M16-2977. [10] YU S, HE J, WU YQ, et al. Clinical observation of Chaihu Guizhi Ganjiang Decoction for treatment of non-alcoholic steatohepatitis of gallbladder heat and spleen cold syndrome[J]. Chin J Integr Tradit West Med Liver Dis, 2023, 33( 6): 509- 511. DOI: 10.3969/j.issn.1005-0264.2023.006.008.余松, 何杰, 吴叶清, 等. 柴胡桂枝干姜汤治疗胆热脾寒型非酒精性脂肪性肝炎的临床观察[J]. 中西医结合肝病杂志, 2023, 33( 6): 509- 511. DOI: 10.3969/j.issn.1005-0264.2023.006.008. [11] ZHOU Y. Clinical observation of using Jiangzhi Xiaozhuo Decoction in the treatment of nonalcoholic steatohepatitis with dampness and turbidity accumulation[J]. J Sichuan Tradit Chin Med, 2023, 41( 1): 141- 144.周云. 降脂消浊汤治疗湿浊蕴积型非酒精性脂肪性肝炎临床疗效观察[J]. 四川中医, 2023, 41( 1): 141- 144. [12] LYU C, SHEN LC, CHEN Z. Curative effect of kuhuang Keli combined with melbine in treating damp-heat accumulation type non-alcoholic steatohepatitis[J]. China J Pharm Econ, 2022, 17( 10): 51- 55. DOI: 10.12010/j.issn.1673-5846.2022.10.010.吕春, 沈兰超, 陈震. 苦黄颗粒联合二甲双胍治疗湿热蕴结型非酒精性脂肪性肝炎的临床效果[J]. 中国药物经济学, 2022, 17( 10): 51- 55. DOI: 10.12010/j.issn.1673-5846.2022.10.010. [13] LONG SS, JIANG W, DONG YN, et al. Clinical observation of self-proposed Xiaopi Huatan Granules combined with silibinin capsules in the treatment of non-alcoholic steatohepatitis with phlegm-damp internal resistance syndrome[J]. Guid J Tradit Chin Med Pharm, 2022, 28( 2): 66- 70.龙爽爽, 姜伟, 董亚楠, 等. 自拟消癖化痰颗粒联合水飞蓟宾胶囊治疗非酒精性脂肪性肝炎(痰湿内阻证)的临床研究[J]. 中医药导报, 2022, 28( 2): 66- 70. [14] LEI YJ, WANG LL, YE XD, et al. Clinical study on modified Chaihu Shugan powder combined with polyene phosphati-dylcholine capsules for nonalcoholic steatohepatitis[J]. J New Chin Med, 2021, 53( 15): 24- 28. DOI: 10.13457/j.cnki.jncm.2021.15.006.雷叶静, 王玲玲, 叶小丹, 等. 柴胡疏肝散加减联合多烯磷脂酰胆碱胶囊治疗非酒精性脂肪性肝炎临床研究[J]. 新中医, 2021, 53( 15): 24- 28. DOI: 10.13457/j.cnki.jncm.2021.15.006. [15] HE QS, LIU Y, XU CJ. Observation of therapeutic effect of Myrrha on non-alcoholic steatohepatitis[J]. Inn Mong J Tradit Chin Med, 2021, 40( 4): 7- 10.何秋硕, 刘尧, 徐春军. 没药治疗非酒精性脂肪性肝炎临床疗效观察[J]. 内蒙古中医药, 2021, 40( 4): 7- 10. [16] CHEN WL, LIANG F, ZHANG YC, et al. Observe on curative effect of Yuzha Quzhi Qinggan prescription combined with glu-tathinone in treating nonalcoholic fatty liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2021, 31( 3): 216- 218. DOI: 10.3969/j.issn.1005-0264.2021.03.008.陈文林, 梁芳, 张云城, 等. 郁楂祛脂清肝方联合谷胱甘肽治疗非酒精性脂肪性肝炎的疗效观察[J]. 中西医结合肝病杂志, 2021, 31( 3): 216- 218. DOI: 10.3969/j.issn.1005-0264.2021.03.008. [17] LEI XBP, LI XY, DAI YN, et al. Clinical observation of self-made zhiyanping granule in the treatment of 45 cases of non-alcoholic steatohepatitis[J]. Chin J Ethnomed Ethnopharmacy, 2021, 30( 5): 84- 87. DOI: 10.3969/j.issn.1007-8517.2021.5.zgmzmjyyzz202105020.雷欣博鹏, 李旭英, 代轶楠, 等. 自拟脂炎平颗粒治疗非酒精性脂肪性肝炎45例临床观察[J]. 中国民族民间医药, 2021, 30( 5): 84- 87. DOI: 10.3969/j.issn.1007-8517.2021.5.zgmzmjyyzz202105020. [18] JIN JJ. Clinical study on Shugan Huazhuo Tang combined with routine western medicine for nonalcoholic steatohepatitis[J]. J New Chin Med, 2021, 53( 5): 83- 86. DOI: 10.13457/j.cnki.jncm.2020.05.021.金晶晶. 舒肝化浊汤联合常规西药治疗非酒精性脂肪性肝炎临床研究[J]. 新中医, 2021, 53( 5): 83- 86. DOI: 10.13457/j.cnki.jncm.2020.05.021. [19] WANG WL, YAN FJ, XU ML, et al. Clinical study on Jiangzhi Tongmai capsules combined with routine therapy for non-alcoholic steatohepatitis[J]. J New Chin Med, 2021, 53( 3): 73- 76. DOI: 10.13457/j.cnki.jncm.2021.03.019.王武玲, 严福建, 徐明丽, 等. 降脂通脉胶囊联合常规疗法治疗非酒精性脂肪性肝炎临床研究[J]. 新中医, 2021, 53( 3): 73- 76. DOI: 10.13457/j.cnki.jncm.2021.03.019. [20] LI YH, YU S, WANG JJ, et al. Effect of Tiaogan Jiangzhi drink on liver function and serum TG and TC levels in patients with nonalcoholic steatohepatitis of phlegm-dampness internal resistance type[J]. Guangming J Chin Med, 2020, 35( 22): 3511- 3513. DOI: 10.3969/j.issn.1003-8914.2020.22.010.李永华, 于收, 王佳佳, 等. 调肝降脂饮对非酒精性脂肪性肝炎痰湿内阻型患者肝功能及血清TG、TC水平的影响[J]. 光明中医, 2020, 35( 22): 3511- 3513. DOI: 10.3969/j.issn.1003-8914.2020.22.010. [21] LIN XM, CHEN S, TANG YQ, et al. Clinical observation of 40 cases of non-alcoholic steatohepatitis treated with spleen deficiency by liver and spleen clearing method[J]. China’s Naturopathy, 2020, 28( 21): 67- 71. DOI: 10.19621/j.cnki.11-3555/r.2020.2127.林雪梅, 陈珊, 唐玉琴, 等. 清肝健脾法治疗脾虚痰瘀互结型非酒精性脂肪性肝炎40例临床观察[J]. 中国民间疗法, 2020, 28( 21): 67- 71. DOI: 10.19621/j.cnki.11-3555/r.2020.2127. [22] HUA P, GUO W, XUE JD, et al. Evaluation the clinic effect for treatment of non-alcoholic steatohepatitis by Jianpi Huazhuo Xiaozhi prescription[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30( 5): 408- 410. DOI: 10.3969/j.issn.1005-0264.2020.05.008.华鹏, 郭薇, 薛敬东, 等. 健脾化浊消脂方治疗非酒精性脂肪性肝炎临床疗效评价[J]. 中西医结合肝病杂志, 2020, 30( 5): 408- 410. DOI: 10.3969/j.issn.1005-0264.2020.05.008. [23] LIU XJ, WANG Y, WANG N, et al. Clinical study on modified Chaishao Liujunzi Decoction in treatment of nonalcoholic steatohepatitis with liver stagnation and spleen deficiency syndrome[J]. Chin J Inf Tradit Chin Med, 2021, 28( 1): 113- 117. DOI: 10.19879/j.cnki.1005-5304.202006364.刘先姜, 王雅, 王娜, 等. 柴芍六君子汤加减治疗非酒精性脂肪性肝炎肝郁脾虚证临床研究[J]. 中国中医药信息杂志, 2021, 28( 1): 113- 117. DOI: 10.19879/j.cnki.1005-5304.202006364. [24] ZHANG XX, LIU YD, YANG C. Clinical study of Baogan Jiedu Decoction retention Enema combined with silybin in the treatment of nonalcoholic steatohepatitis[J]. Acta Chin Med Pharmacol, 2020, 48( 10): 47- 51. DOI: 10.19664/j.cnki.1002-2392.200177.张晓星, 刘延东, 杨川. 保肝解毒汤保留灌肠联合水飞蓟宾治疗非酒精性脂肪性肝炎的临床研究[J]. 中医药学报, 2020, 48( 10): 47- 51. DOI: 10.19664/j.cnki.1002-2392.200177. [25] LI SX, YUAN XX, LI DD, et al. Effects of Lianlou Hugan Decoction on oxidative stress and inflammatory factors in patients with non-alcoholic steatohepatitis[J]. J Hunan Univ Chin Med, 2020, 40( 7): 892- 896. DOI: 10.3969/j.issn.1674-070X.2020.07.022.李硕熙, 袁星星, 李丹丹, 等. 连蒌护肝汤对非酒精性脂肪性肝炎氧化应激及炎性因子水平的影响[J]. 湖南中医药大学学报, 2020, 40( 7): 892- 896. DOI: 10.3969/j.issn.1674-070X.2020.07.022. [26] ZHANG XX, YANG C, LIU YD, et al. The effect of Baogan Jiedu Decoction Enema on nonalcoholic steatohepatitis[J]. China Health Stand Manag, 2020, 11( 13): 100- 102. DOI: 10.3969/j.issn.1674-9316.2020.13.042.张晓星, 杨川, 刘延东, 等. 保肝解毒汤灌肠治疗非酒精性脂肪性肝炎的效果[J]. 中国卫生标准管理, 2020, 11( 13): 100- 102. DOI: 10.3969/j.issn.1674-9316.2020.13.042. [27] XUE XX, XIE CE. Clinical effect and mechanisms of Yinchen Linggui Zhugan Decoction in treatment of non-alcoholic steatohepatitis[J]. China Med, 2020, 15( 6): 907- 911. DOI: 10.3760/j.issn.1673-4777.2020.06.025.薛晓轩, 谢春娥. 茵陈苓桂术甘汤治疗非酒精性脂肪性肝炎的临床效果及作用机制[J]. 中国医药, 2020, 15( 6): 907- 911. DOI: 10.3760/j.issn.1673-4777.2020.06.025. [28] ZHOU Y, ZHANG B. Curative effect of using Jianpi Huotan Decoction combined with western medicine in the treatment of non-alcoholic hepatitis of spleen-deficiency phlegm-stagnation syndrome[J]. J Sichuan Tradit Chin Med, 2020, 38( 4): 124- 127.周燕, 张冰. 健脾豁痰汤联合西药治疗脾虚痰阻证非酒精性肝炎疗效观察[J]. 四川中医, 2020, 38( 4): 124- 127. [29] LUO MC, XUE XX, CHENG XF, et al. Clinical study on the effect of Chaihu Shugan Powder on non-alcoholic steatohepatitis inflammatory factors[J]. J Tianjin Univ Tradit Chin Med, 2020, 37( 2): 187- 192. DOI: 10.11656/j.issn.1672-1519.2020.02.16.雒明池, 薛晓雪, 程旭峰, 等. 柴胡疏肝散对非酒精性脂肪性肝炎炎症因子影响的临床研究[J]. 天津中医药, 2020, 37( 2): 187- 192. DOI: 10.11656/j.issn.1672-1519.2020.02.16. [30] ZHOU HJ, CHEN G, XIE HD. Clinical evaluation of Shen Ze Shugan capsule in the treatment of non-alcoholic steatohepatitis(damp-heat stagnation syndrome)[J]. Tradit Chin Drug Res Clin Pharmacol, 2019, 30( 9): 1133- 1137. DOI: 10.19378/j.issn.1003-9783.2019.09.019.周海娟, 陈刚, 谢红丹. 参泽舒肝胶囊对非酒精性脂肪性肝炎湿热瘀结证作用研究[J]. 中药新药与临床药理, 2019, 30( 9): 1133- 1137. DOI: 10.19378/j.issn.1003-9783.2019.09.019. [31] ZHANG SY, ZHU TB, WU Q. Clinical study on the treatment of non-alcoholic steatohepatitis by resolving phlegm, removing dampness and activating blood circulation[J/CD]. Electron J Gen Stomatol, 2019, 6( 30): 159- 160.张耸阳, 朱铁兵, 吴倩. 化痰祛湿活血方治疗非酒精性脂肪性肝炎临床研究[J/CD]. 全科口腔医学杂志(电子版), 2019, 6( 30): 159- 160. [32] ZOU JF. Effect of Shengjiang Decoction on liver function and therapeutic effect in patients with non-alcoholic steatohepatitis of liver depression and spleen deficiency type[J]. J Pract Tradit Chin Intern Med, 2019, 33( 9): 57- 59. DOI: 10.13729/j.issn.1671-7813.Z20190258.邹俊锋. 升降汤对肝郁脾虚型非酒精性脂肪性肝炎患者肝功能及疗效的影响[J]. 实用中医内科杂志, 2019, 33( 9): 57- 59. DOI: 10.13729/j.issn.1671-7813.Z20190258. [33] WANG SJ, WANG YJ. Clinical efficacy and safety of lipid-lowering therapy in treating steatohepatitis[J]. Chin J Gerontol, 2019, 39( 10): 2390- 2392. DOI: 10.3969/j.issn.1005-9202.2019.10.031.王思鉴, 王雅君. 清肝降脂合剂治疗痰瘀互结型脂肪性肝炎的临床疗效及安全性[J]. 中国老年学杂志, 2019, 39( 10): 2390- 2392. DOI: 10.3969/j.issn.1005-9202.2019.10.031. [34] LAN XM, WU YN, CHEN SD, et al. Treatment of nonalcoholic steatohepatitis by Zaozhu Yinchen recipe[J]. Chin J Integr Tradit West Med, 2019, 39( 5): 557- 560. DOI: 10.7661/j.cjim.20190125.099.兰秀梅, 吴耀南, 陈少东, 等. 皂术茵陈方治疗非酒精性脂肪性肝炎的临床观察[J]. 中国中西医结合杂志, 2019, 39( 5): 557- 560. DOI: 10.7661/j.cjim.20190125.099. [35] TANG Y, LI J. Observation of integrated Chinese and Western medicine in the treatment of non-alcoholic steatohepatitis[J]. J Pract Tradit Chin Med, 2019, 35( 4): 454- 455.唐燕, 李洁. 中西医结合治疗非酒精性脂肪性肝炎疗效观察[J]. 实用中医药杂志, 2019, 35( 4): 454- 455. [36] FAN YW, XIE WP. Effect of soothing liver and strengthening spleen therapy in treating non-alcoholic steatohepatitis and analysis of changes in BMI and AC[J]. Chin Med Mod Distance Educ China, 2018, 16( 23): 57- 58. DOI: 10.3969/j.issn.1672-2779.2018.23.024.范云万, 谢维鹏. 疏肝健脾法治疗非酒精性脂肪性肝炎效果及对BMI及AC的变化分析[J]. 中国中医药现代远程教育, 2018, 16( 23): 57- 58. DOI: 10.3969/j.issn.1672-2779.2018.23.024. [37] LIU JR, ZHU XN, WANG J, et al. Clinical evaluation of eliminating phlegm and activating blood granule in treating nonalcoholic steatohepatitis with syndrome of phlegm and blood stasis[J]. World Latest Med Inf, 2018, 18( 98): 28- 30. DOI: 10.19613/j.cnki.1671-3141.2018.98.010.刘菊容, 朱晓宁, 汪静, 等. 祛痰活血颗粒治疗非酒精性脂肪性肝炎痰瘀互结证的临床疗效评价[J]. 世界最新医学信息文摘, 2018, 18( 98): 28- 30. DOI: 10.19613/j.cnki.1671-3141.2018.98.010. [38] ZHENG N, DAI M. Clinical study on modified Gexia Zhuyu Tang combined with Erchen Tang for nonalcoholic steatohepatitis[J]. J New Chin Med, 2018, 50( 11): 98- 101. DOI: 10.13457/j.cnki.jncm.2018.11.027.郑娜, 戴孟. 膈下逐瘀汤合二陈汤加减治疗非酒精性脂肪性肝炎临床研究[J]. 新中医, 2018, 50( 11): 98- 101. DOI: 10.13457/j.cnki.jncm.2018.11.027. [39] LI J. Clinical observation on treatment for nonalcoholic steatohepatitis with self-set hypolipidemic decoction[J]. Guangxi J Tradit Chin Med, 2018, 41( 5): 6- 9. DOI: 10.3969/j.issn.1003-0719.2018.05.003.李洁. 自拟降脂汤治疗非酒精性脂肪性肝炎疗效观察[J]. 广西中医药, 2018, 41( 5): 6- 9. DOI: 10.3969/j.issn.1003-0719.2018.05.003. [40] WU D, LI JX, XIE CE, et al. Observing the effect of Yinchen Linggui prescription on NASH based on liver ultrasonic attenuation parameters[J]. Chin J Integr Trad West Med Dig, 2018, 26( 10): 817- 822. DOI: 10.3969/j.issn.1671-038X.2018.10.03.吴迪, 李军祥, 谢春娥, 等. 基于肝脏超声衰减参数观察茵陈苓桂剂干预非酒精性脂肪性肝炎的作用研究[J]. 中国中西医结合消化杂志, 2018, 26( 10): 817- 822. DOI: 10.3969/j.issn.1671-038X.2018.10.03. [41] CAI Z, MAI JY. Clinical effects of Shenge Granules on senile non-alcoholic steatohepatitis due to spleen deficiency and phlegm turbidity[J]. Chin Tradit Pat Med, 2018, 40( 9): 1935- 1938. DOI: 10.3969/j.issn.1001-1528.2018.09.008.蔡峥, 麦静愔. 参葛颗粒对脾虚痰浊证老年性非酒精性脂肪性肝炎患者的临床疗效[J]. 中成药, 2018, 40( 9): 1935- 1938. DOI: 10.3969/j.issn.1001-1528.2018.09.008. [42] HU ZB, LIU LL, CHEN YH, et al. Clinical observation on particles of Shugan Jiangzhi Granules in treating non-alcoholic fatty liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2018, 28( 4): 208- 210. DOI: 10.3969/j.issn.1005-0264.2018.04.006.胡振斌, 柳琳琳, 陈永洪, 等. 疏肝降脂颗粒治疗非酒精性脂肪性肝炎临床观察[J]. 中西医结合肝病杂志, 2018, 28( 4): 208- 210. DOI: 10.3969/j.issn.1005-0264.2018.04.006. [43] GU HP, MA WM, KANG NS, et al. Clinical effectiveness and influence of Jieyuxiaozhi formula on life quality in patients with non-alcoholic steatohepatitis[J]. China Mod Dr, 2018, 56( 23): 137- 140, 144.谷红苹, 马伟明, 康年松, 等. 解郁消脂方对非酒精性脂肪性肝炎的临床疗效及生活质量的影响[J]. 中国现代医生, 2018, 56( 23): 137- 140, 144. [44] LI HS, YING H, HE XL, et al. Clinical observation of Qutan Huoxue Decoction for treating non-alcoholic steatohepatitis[J]. Chin Arch Tradit Chin Med, 2018, 36( 7): 1554- 1556. DOI: 10.13193/j.issn.1673-7717.2018.07.004.李红山, 应豪, 贺晓立, 等. 祛痰活血方治疗非酒精性脂肪性肝炎的临床观察[J]. 中华中医药学刊, 2018, 36( 7): 1554- 1556. DOI: 10.13193/j.issn.1673-7717.2018.07.004. [45] LI CQ, KE XX. Clinical effect of Jiangzhi Huoxue Decoction combined with polyene Phos-phatidylcholine on nonalcoholic steatohepatitis[J]. China Foreign Med Treatment, 2018, 37( 15): 25- 26, 29. DOI: 10.16662/j.cnki.1674-0742.2018.15.025.李超群, 柯欣欣. 降脂活血汤联合多烯磷脂酰胆碱治疗非酒精性脂肪性肝炎的临床效果[J]. 中外医疗, 2018, 37( 15): 25- 26, 29. DOI: 10.16662/j.cnki.1674-0742.2018.15.025. [46] CHEN X, ZHANG JF, SU WD, et al. Clinical study of self-made Chinese medicines in treating non-alcoholic steatohepatitis of the Pixu Tanshi type[J]. Chin J Integr Tradit West Med Liver Dis, 2018, 28( 2): 82- 84. DOI: 10.3969/j.issn.1005-0264.2018.02.005.陈欣, 张俊富, 苏文弟, 等. 自拟中药治疗脾虚痰湿型非酒精性脂肪性肝炎的临床研究[J]. 中西医结合肝病杂志, 2018, 28( 2): 82- 84. DOI: 10.3969/j.issn.1005-0264.2018.02.005. [47] MAI JY, GAO YQ, CAI Z, et al. Shenge Recipe in the treatment of senile non-alcoholic steatohepatitis with syndrome of phlegm turbidity due to spleen deficiency: A randomized, double-blind and placebo-controlled trial[J]. Shanghai J Tradit Chin Med, 2018, 52( 4): 44- 48. DOI: 10.16305/j.1007-1334.2018.04.013.麦静愔, 高月求, 蔡峥, 等. 参葛方治疗脾虚痰浊型老年性非酒精性脂肪性肝炎的随机、双盲、安慰剂对照研究[J]. 上海中医药杂志, 2018, 52( 4): 44- 48. DOI: 10.16305/j.1007-1334.2018.04.013. [48] WANG H, ZHANG W. Clinical observation of 100 cases on“Danshao Shugan Granules” combined with“Silibinin capsules” in the treatment of humid heat and spleen deficiency type of nonalcoholic steatohepatitis[J]. Chin J Integr Tradit West Med Liver Dis, 2018, 28( 1): 14- 16. DOI: 10.3969/j.issn.1005-0264.2018.01.005.王慧, 张玮. 丹芍疏肝颗粒联合水飞蓟宾胶囊治疗湿热内蕴兼肝郁脾虚型非酒精性脂肪性肝炎临床研究[J]. 中西医结合肝病杂志, 2018, 28( 1): 14- 16. DOI: 10.3969/j.issn.1005-0264.2018.01.005. [49] WU DH, JU Y. Clinical observation on soothing liver and strengthening spleen prescription in the treatment of nonalcoholic steatohepatitis[J]. Guangming J Chin Med, 2018, 33( 2): 201- 202. DOI: 10.3969/j.issn.1003-8914.2018.02.022.吴东辉, 鞠莹. 疏肝健脾方治疗非酒精性脂肪性肝炎的临床观察[J]. 光明中医, 2018, 33( 2): 201- 202. DOI: 10.3969/j.issn.1003-8914.2018.02.022. [50] XIAO DM, LIU YJ. Clinical observation of Chaige Jiangzhi Drink in the treatment of non alcoholic fatty hepatitis[J]. Diet Health, 2021( 38): 19- 20.肖德梅, 刘益军. 柴葛降脂饮治疗非酒精性脂肪性肝炎的临床观察[J]. 饮食保健, 2021( 38): 19- 20. [51] ZHANG DL, SUN C, CHEN Y. Clinical observation on the treatment of non-alcoholic fatty liver disease with spleen deficiency and phlegm obstruction by tonifying the spleen and clearing the liver[J]. Med Health, 2021( 7): 138- 140.张东兰, 孙超, 陈颜. 扶脾清肝治疗脾虚痰阻型非酒精性脂肪性肝炎的临床观察[J]. 医药卫生, 2021( 7): 138- 140. [52] ZHANG XX, NIU DL. The effect of Peitu Qingyu Formula on liver function and fatty liver degree in non alcoholic hepatitis patients with spleen deficiency and phlegm obstruction syndrome[J]. Sichuan J Physiol Sci, 2021, 43( 11): 1964- 1965, 1979.张小旭, 牛栋良. 培土清瘀方对脾虚痰阻证非酒精性肝炎患者肝功能及脂肪肝程度的影响[J]. 四川生理科学杂志, 2021, 43( 11): 1964- 1965, 1979. [53] LIN L, LIANG HQ, ZHUANG HL, et al. Effect of Zaozhu Yinchen recipe on intestinal flora in treatment of nonalcoholic steatohepatitis[J]. Chin J Integr Trad West Med, 2018, 38( 6): 673- 676. DOI: 10.7661/j.cjim.20180119.103.林立, 梁惠卿, 庄鸿莉, 等. 皂术茵陈方治疗非酒精性脂肪性肝炎的临床观察及其对肠道菌群的影响[J]. 中国中西医结合杂志, 2018, 38( 6): 673- 676. DOI: 10.7661/j.cjim.20180119.103. [54] LI YL. Observation on the therapeutic effect of Qinggan Huatan Huoxue Formula in the treatment of non-alcoholic steatohepatitis[J]. Med Health, 2022( 7): 79- 82.李艳丽. 论清肝化痰活血方治疗非酒精性脂肪性肝炎的疗效观察[J]. 医药卫生, 2022( 7): 79- 82. [55] ABDULRAHEEM S, BONDEMARK L. The reporting of blinding in orthodontic randomized controlled trials: Where do we stand?[J]. Eur J Orthod, 2019, 41( 1): 54- 58. DOI: 10.1093/ejo/cjy021. [56] SHAO JY, HU YH, YUAN WA, et al. Research progress on preparation technology and evaluation methods of Chinese medicine placebo[J]. Drug Eval Res, 2023, 46( 5): 1125- 1130. DOI: 10.7501/j.issn.1674-6376.2023.05.024.邵靖渊, 胡薏慧, 元唯安, 等. 中药安慰剂制作工艺及评价方法的研究进展[J]. 药物评价研究, 2023, 46( 5): 1125- 1130. DOI: 10.7501/j.issn.1674-6376.2023.05.024. [57] ZHANG Y, WANG JH, HU YY, et al. Determining sample size of pilot trials in traditional Chinese medicine[J]. J Tradit Chin Med, 2021, 62( 4): 307- 311. DOI: 10.13288/j.11-2166/r.2021.04.008.张颖, 王俊慧, 胡烨胤, 等. 中医药临床研究中预试验样本量的确定[J]. 中医杂志, 2021, 62( 4): 307- 311. DOI: 10.13288/j.11-2166/r.2021.04.008. [58] YAO WW, LIU KJ, JI J, et al. Situation analysis of the clinical outcomes of randomized controlled trials of traditional Chinese medicine intervention in non-alcoholic fatty liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2024, 34( 2): 136- 139. DOI: 10.3969/j.issn.1005-0264.2024.002.009.姚伟伟, 刘可佳, 吉静, 等. 中医药干预非酒精性脂肪性肝病随机对照临床研究结局指标的现状分析[J]. 中西医结合肝病杂志, 2024, 34( 2): 136- 139. DOI: 10.3969/j.issn.1005-0264.2024.002.009. [59] CHEN Y, LU S, XU XW, et al. Interpretation of key points of the guideline for clinical trials of drugs for nonalcoholic steatohepatitis(trial)[J]. Chin J Clin Pharmacol, 2020, 36( 3): 379- 384. DOI: 10.13699/j.cnki.1001-6821.2020.03.045.陈颖, 鲁爽, 徐小文, 等.《非酒精性脂肪性肝炎治疗药物临床试验原则(试行)》要点解读[J]. 中国临床药理学杂志, 2020, 36( 3): 379- 384. DOI: 10.13699/j.cnki.1001-6821.2020.03.045. -



 PDF下载 ( 1291 KB)
PDF下载 ( 1291 KB)

 下载:
下载:



