灵芝酸A对刀豆球蛋白A诱导的急性免疫性肝损伤小鼠模型的影响及其作用机制
DOI: 10.12449/JCH241211
Effect of ganoderic acid A on a mouse model of concanavalin A-induced acute immune liver injury and its mechanism
-
摘要:
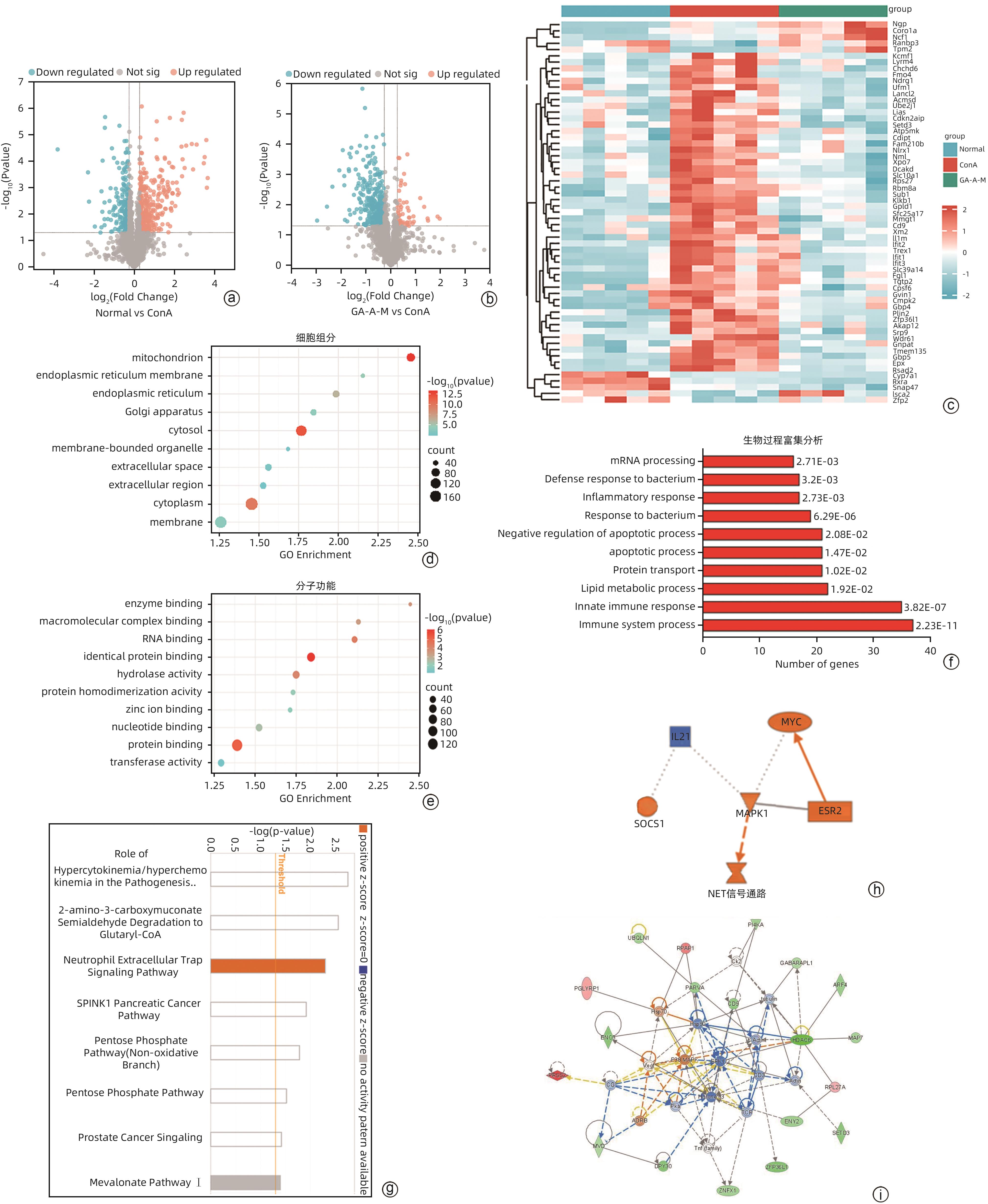
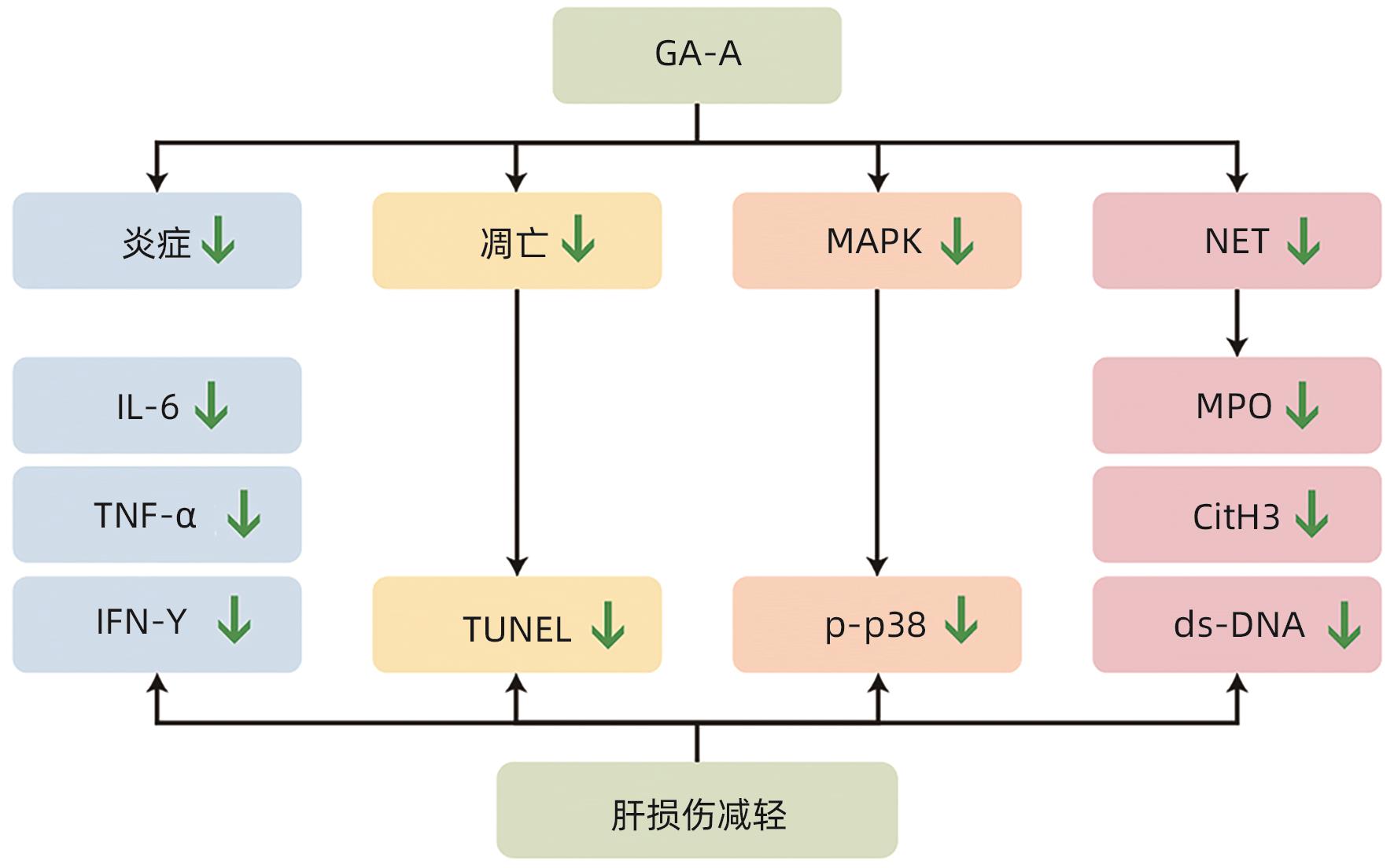
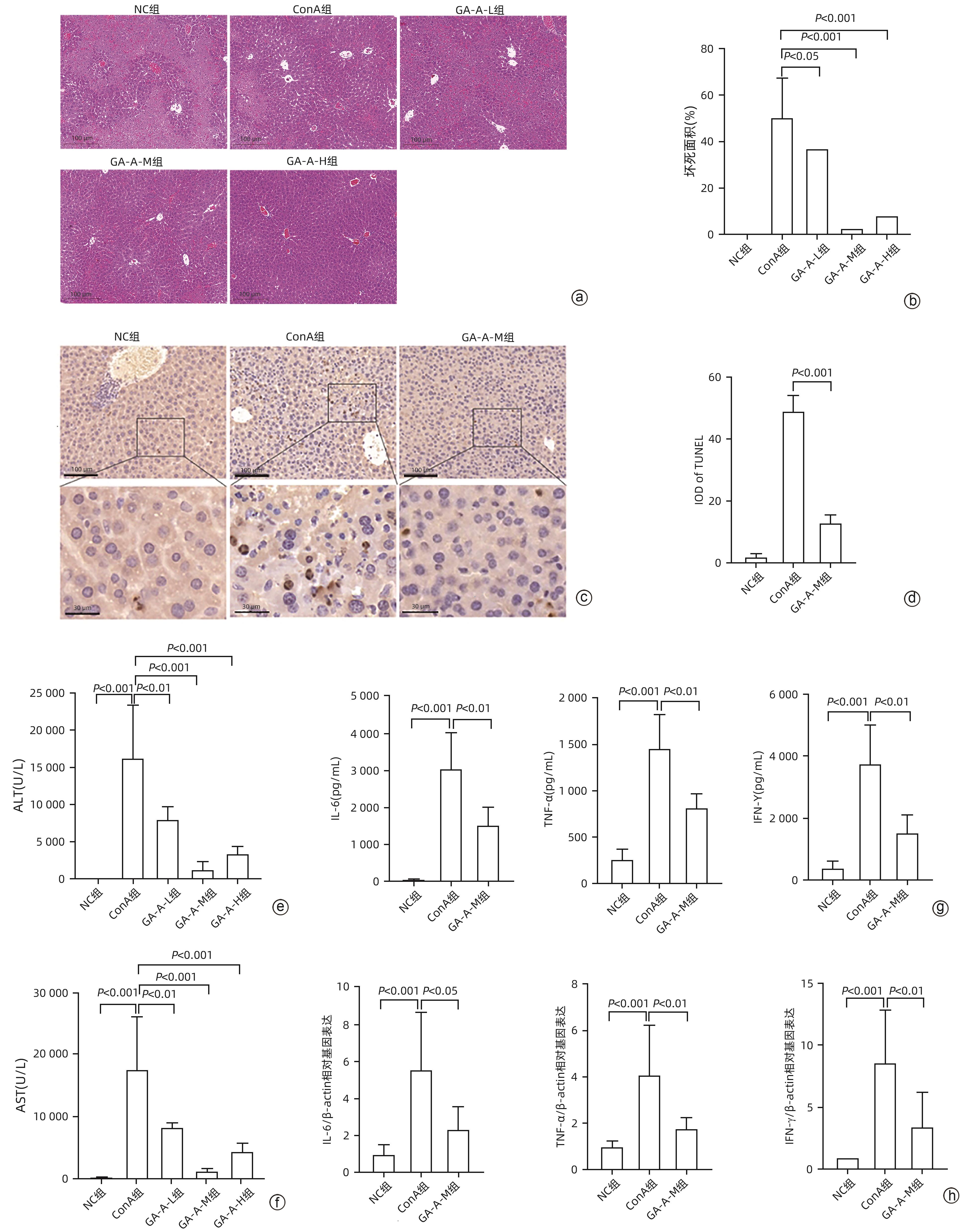
目的 观察灵芝酸A(GA-A)对刀豆球蛋白A(ConA)诱导的小鼠自身免疫性肝炎(AIH)模型的治疗作用。 方法 将35只小鼠随机分为空白组(NC组)、模型组(ConA组)和GA-A低、中、高剂量治疗组(GA-A-L组、GA-A-M组、GA-A-H组),每组各7只小鼠。通过尾静脉注射ConA建立经典AIH小鼠模型,1 h后通过腹腔注射不同剂量GA-A治疗。应用蛋白质组学技术探究GA-A对肝细胞的保护机制,另外在体外用全反式维甲酸(ATRA)将HL-60细胞分化为dHL-60中性粒细胞来验证GA-A的作用机制。检测炎症(血清ALT和AST活性、HE染色及炎症相关基因)、凋亡(TUNEL染色)、中性粒细胞和中性粒细胞胞外陷阱(NET)标志物[髓过氧化物酶(MPO)、瓜氨酸化组蛋白3(CitH3)、Ly6G、游离双链DNA(dsDNA)]及p38磷酸化指标。计量资料两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与NC组相比,ConA组小鼠血清ALT和AST水平显著增加(P值均<0.001)。与ConA组相比,GA-A治疗显著降低ALT和AST水平(P值均<0.01)。HE染色结果表明,ConA组小鼠肝脏发生明显坏死。GA-A治疗后显著减少肝坏死面积和TUNEL阳性肝细胞的数量(P值均<0.05)。另外,与ConA组相比,GA-A治疗后血清和肝组织中炎症因子IL-6、TNF-α和IFN-γ表达水平显著降低(P值均<0.05)。蛋白质组学分析提示,GA-A通过抑制NET的释放和p38 MAPK通路来减轻ConA诱导的急性免疫性肝损伤。小鼠肝组织免疫荧光染色结果显示,与ConA组相比,GA-A治疗组MPO阳性中性粒细胞的数量及Ly6G和CitH3阳性细胞的数量显著降低(P值均<0.01)。Western Blot及dsDNA结果显示,GA-A显著抑制小鼠肝组织及dHL-60细胞中的NET标志物dsDNA、CitH3以及p38磷酸化水平(P值均<0.05)。 结论 GA-A抑制p38 MAPK通路和NET释放减轻肝脏炎症反应和肝细胞死亡,从而减轻ConA诱导的急性免疫性肝损伤。 本研究为GA-A通过调节嗜中性粒细胞功能治疗免疫性肝损伤提供了理论依据。 Abstract:Objective To investigate the therapeutic effect of ganoderic acid A (GA-A) on a mouse model of concanavalin A (ConA)-induced autoimmune hepatitis (AIH). Methods A total of 35 mice were randomly divided into control group (NC group), model group (ConA group), and low-, middle-, and high-dose GA-A treatment groups (GA-A-L, GA-A-M, and GA-A-H groups, respectively), with 7 mice in each group. ConA was injected via the caudal vein of mice to establish a classic mouse model of AIH, and different doses of GA-A were administered via intraperitoneal injection 1 hour later for treatment. Proteomic techniques were used to investigate the protective mechanism of GA-A on hepatocytes, and HL-60 cells were differentiated into dHL-60 neutrophils by all-trans retinoic acid in vitro to validate the mechanism of action of GA-A. Related indicators were measured, including inflammatory markers (the activities of serum alanine aminotransferase [ALT] and aspartate aminotransferase [AST], HE staining, and inflammation-related genes), apoptosis markers (TUNEL staining), neutrophils, and neutrophil extracellular trap (NET) markers (myeloperoxidase [MPO], citrullinated histone H3 [CitH3], Ly6G, and free double-stranded DNA [dsDNA]), and p38 phosphorylation markers. The independent samples t-test was used for comparison of continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the NC group, the ConA group had significant increases in the serum levels of ALT and AST (both P<0.001), and compared with the ConA group, GA-A treatment significantly reduced the levels of ALT and AST (both P<0.01). HE staining showed that the mice in the ConA group had significant liver necrosis, while GA-A treatment significantly reduced the area of liver necrosis and the number of TUNEL-positive cells (both P<0.05). Compared with the ConA group, the GA-A group had significant reductions in the expression levels of the inflammatory factors interleukin-6, tumor necrosis factor-α, and interferon gamma in serum and liver tissue (all P<0.05). The proteomic analysis showed that GA-A alleviated ConA-induced acute immune liver injury by inhibiting the release of NET and the p38 MAPK pathway. Immunofluorescent staining of mouse liver tissue showed that compared with the ConA group, the GA-A group had significant reductions in the number of MPO-positive neutrophils and the number of cells with positive Ly6G and CitH3 (all P<0.01). Western Blot and dsDNA testing showed that GA-A significantly inhibited the levels of the NET markers dsDNA and CitH3 and the level of p38 phosphorylation in liver tissue and dHL-60 cells (all P<0.05). Conclusion GA-A alleviates liver inflammatory response and hepatocyte death by inhibiting the p38 MAPK pathway and the release of NET, thereby alleviating ConA-induced acute immune liver injury. This study provides a theoretical basis for the use of GA-A to treat immune liver injury by regulating neutrophil function. -
Key words:
- Ganoderic Acid A /
- Concanavalin A /
- Hepatitis, Autoimmune /
- Extracellular Traps /
- Proteomics /
- Mice, Inbred C57BL
-
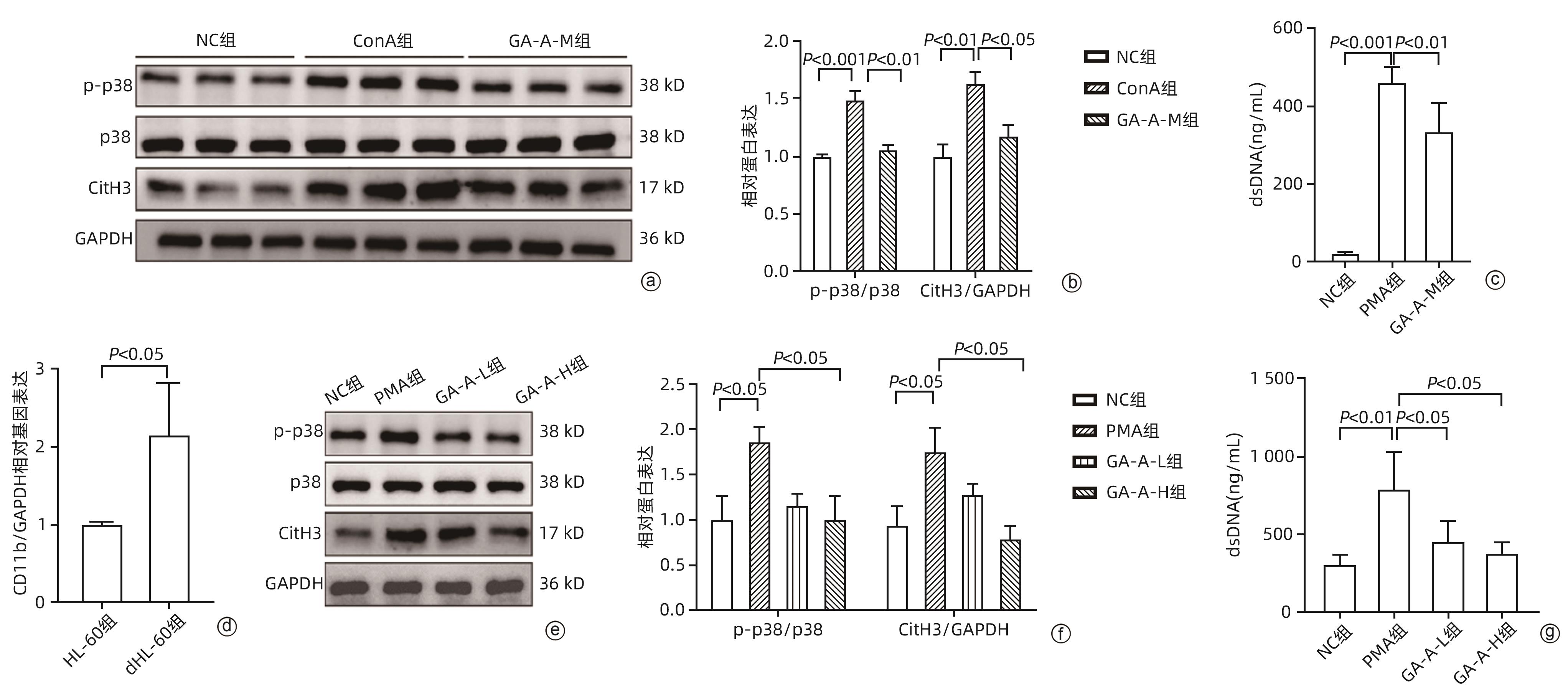
注: a,Western Blot检测3组小鼠肝脏p-p38、p38、CitH3和GAPDH的蛋白表达;b, p-p38/p38 和 CitH3/GAPPH 的蛋白表达水平定量;c,3组小鼠血清dsDNA水平;d,HL-60和dHL-60细胞中CD11b的相对mRNA表达水平;e,Western Blot检测4组中dHL-60细胞的p-p38、p38、CitH3和GAPDH的蛋白表达;f,4组p-p38/p38 和 CitH3/GAPPH 的蛋白表达水平定量;g,4组中dHL-60细胞上清液中dsDNA 水平。
图 4 GA-A 通过抑制 p38 磷酸化来抑制 NET 形成
Figure 4. GA-A inhibits NET formation by inhibiting p38 phosphorylation
表 1 引物序列
Table 1. Primer sequences
基因 正向(5'-3') 反向(5'-3') β-actin GTGCTATGTTGCTCTAGACTTCG ATGCCACAGGATTCCATACC GAPDH CCTCTATGCCAACACAGT AGCCACCAATCCACACAG IL-6 GCTACCAAACTGGATATAATCAGGA CCAGGTAGCTATGGTACTCCAGAA TNF-α CTGAACTTCGGGGTGATCGG GGCTTGTCACTCGAATTTTGAGA IFN-γ ATGAACGCTACACACTGCATC CCATCCTTTTGCCAGTTCCTC CD11b ATGGACGCTGATGGCAATACC TCCCCATTCACGTCTCCCA -
[1] CHUNG YY, RAHIM MN, HENEGHAN MA. Autoimmune hepatitis and pregnancy: Considerations for the clinician[J]. Expert Rev Clin Immunol, 2022, 18( 4): 325- 333. DOI: 10.1080/1744666X.2022.2044307. [2] SMOLKA V, TKACHYK O, EHRMANN J, et al. Acute onset of autoimmune hepatitis in children and adolescents[J]. Hepatobiliary Pancreat Dis Int, 2020, 19( 1): 17- 21. DOI: 10.1016/j.hbpd.2019.08.004. [3] SHARMA R, VERNA EC, SÖDERLING J, et al. Increased mortality risk in autoimmune hepatitis: A nationwide population-based cohort study with histopathology[J]. Clin Gastroenterol Hepatol, 2021, 19( 12): 2636- 2647. DOI: 10.1016/j.cgh.2020.10.006. [4] TRIVEDI PJ, HIRSCHFIELD GM. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases[J]. Gut, 2021, 70( 10): 1989- 2003. DOI: 10.1136/gutjnl-2020-322362. [5] SIRBE C, SIMU GL, SZABO I, et al. Pathogenesis of autoimmune hepatitis-cellular and molecular mechanisms[J]. Int J Mol Sci, 2021, 22( 24): 13578. DOI: 10.3390/ijms222413578. [6] WEI YR, LI YM, YAN L, et al. Alterations of gut microbiome in autoimmune hepatitis[J]. Gut, 2020, 69( 3): 569- 577. DOI: 10.1136/gutjnl-2018-317836. [7] DYSON JK, DE MARTIN E, DALEKOS GN, et al. Review article: Unanswered clinical and research questions in autoimmune hepatitis-conclusions of the International Autoimmune Hepatitis Group Research Workshop[J]. Aliment Pharmacol Ther, 2019, 49( 5): 528- 536. DOI: 10.1111/apt.15111. [8] BRINKMANN V, REICHARD U, GOOSMANN C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303( 5663): 1532- 1535. DOI: 10.1126/science.1092385. [9] CASTANHEIRA FVS, KUBES P. Neutrophils and NET in modulating acute and chronic inflammation[J]. Blood, 2019, 133( 20): 2178- 2185. DOI: 10.1182/blood-2018-11-844530. [10] MASUDA S, NAKAZAWA D, SHIDA H, et al. NETosis markers: Quest for specific, objective, and quantitative markers[J]. Clin Chim Acta, 2016, 459: 89- 93. DOI: 10.1016/j.cca.2016.05.029. [11] TANAKA K, KOIKE Y, SHIMURA T, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model[J]. PLoS One, 2014, 9( 11): e111888. DOI: 10.1371/journal.pone.0111888. [12] YIPP BG, KUBES P. NETosis: How vital is it?[J]. Blood, 2013, 122( 16): 2784- 2794. DOI: 10.1182/blood-2013-04-457671. [13] LOOD C, BLANCO LP, PURMALEK MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease[J]. Nat Med, 2016, 22( 2): 146- 153. DOI: 10.1038/nm.4027. [14] HAN F, DING ZF, SHI XL, et al. Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice[J]. Redox Biol, 2023, 64: 102787. DOI: 10.1016/j.redox.2023.102787. [15] KESHARI RS, VERMA A, BARTHWAL MK, et al. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NET release from human neutrophils[J]. J Cell Biochem, 2013, 114( 3): 532- 540. DOI: 10.1002/jcb.24391. [16] ZHAO XH, YANG L, CHANG N, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling[J]. Cell Death Dis, 2020, 11( 5): 379. DOI: 10.1038/s41419-020-2582-1. [17] RADWAN FFY, HOSSAIN A, GOD JM, et al. Reduction of myeloid-derived suppressor cells and lymphoma growth by a natural triterpenoid[J]. J Cell Biochem, 2015, 116( 1): 102- 114. DOI: 10.1002/jcb.24946. [18] CHI BJ, WANG SQ, BI S, et al. Effects of ganoderic acid A on lipopolysaccharide-induced proinflammatory cytokine release from primary mouse microglia cultures[J]. Exp Ther Med, 2018, 15( 1): 847- 853. DOI: 10.3892/etm.2017.5472. [19] CHENG Y, XIE P. Ganoderic acid A holds promising cytotoxicity on human glioblastoma mediated by incurring apoptosis and autophagy and inactivating PI3K/AKT signaling pathway[J]. J Biochem Mol Toxicol, 2019, 33( 11): e22392. DOI: 10.1002/jbt.22392. [20] XU LX, YAN LJ, HUANG SP. Ganoderic acid A against cyclophosphamide-induced hepatic toxicity in mice[J]. J Biochem Mol Toxicol, 2019, 33( 4): e22271. DOI: 10.1002/jbt.22271. [21] MA JQ, ZHANG YJ, TIAN ZK. Anti-oxidant, anti-inflammatory and anti-fibrosis effects of ganoderic acid A on carbon tetrachloride induced nephrotoxicity by regulating the Trx/TrxR and JAK/ROCK pathway[J]. Chem Biol Interact, 2021, 344: 109529. DOI: 10.1016/j.cbi.2021.109529. [22] TADOKORO T, MORISHITA A, SAKAMOTO T, et al. Galectin-9 ameliorates fulminant liver injury[J]. Mol Med Rep, 2017, 16( 1): 36- 42. DOI: 10.3892/mmr.2017.6606. [23] RANI R, TANDON A, WANG J, et al. Stellate cells orchestrate concanavalin A-induced acute liver damage[J]. Am J Pathol, 2017, 187( 9): 2008- 2019. DOI: 10.1016/j.ajpath.2017.05.015. [24] WANG KC, WU WR, JIANG XW, et al. Multi-omics analysis reveals the protection of gasdermin D in concanavalin A-induced autoimmune hepatitis[J]. Microbiol Spectr, 2022, 10( 5): e0171722. DOI: 10.1128/spectrum.01717-22. [25] XU HL, LIU LL, CHEN YX, et al. The chemical character of polysaccharides from processed Morindae officinalis and their effects on anti-liver damage[J]. Int J Biol Macromol, 2019, 141: 410- 421. DOI: 10.1016/j.ijbiomac.2019.08.213. [26] HE GW, GÜNTHER C, KREMER AE, et al. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury[J]. Gut, 2017, 66( 4): 716- 723. DOI: 10.1136/gutjnl-2015-311247. [27] LI XH, ZHANG YT, WANG JP, et al. zVAD alleviates experimental autoimmune hepatitis in mice by increasing the sensitivity of macrophage to TNFR1-dependent necroptosis[J]. J Autoimmun, 2022, 133: 102904. DOI: 10.1016/j.jaut.2022.102904. [28] LIU FL, SHI KJ, DONG JJ, et al. Ganoderic acid A attenuates high-fat-diet-induced liver injury in rats by regulating the lipid oxidation and liver inflammation[J]. Arch Pharm Res, 2020, 43( 7): 744- 754. DOI: 10.1007/s12272-020-01256-9. [29] SAFFARZADEH M, JUENEMANN C, QUEISSER MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones[J]. PLoS One, 2012, 7( 2): e32366. DOI: 10.1371/journal.pone.0032366. [30] REN AH, DONG YB, MIAO RZ, et al. Effect of ganoderic acid A on glycolysis and its key rate-limiting enzymes of non-small cell lung cancer PC9 cells[J]. J Jilin Univ(Med Ed), 2024, 50( 3): 682- 688. DOI: 10.13481/j.1671-587X.20240312.任爱华, 董彦伯, 苗润芝, 等. 灵芝酸A对非小细胞肺癌PC9细胞糖酵解及其关键限速酶的影响[J]. 吉林大学学报(医学版), 2024, 50( 3): 682- 688. DOI: 10.13481/j.1671-587X.20240312. [31] LV XC, WU Q, CAO YJ, et al. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition[J]. Food Funct, 2022, 13( 10): 5820- 5837. DOI: 10.1039/d1fo03219d. [32] ZHU J, DING JX, LI SY, et al. Ganoderic acid A ameliorates non-alcoholic streatohepatitis(NASH) induced by high-fat high-cholesterol diet in mice[J]. Exp Ther Med, 2022, 23( 4): 308. DOI: 10.3892/etm.2022.11237. [33] MA F, CHANG XJ, WANG GY, et al. Streptococcus suis serotype 2 stimulates neutrophil extracellular traps formation via activation of p38 MAPK and ERK1/2[J]. Front Immunol, 2018, 9: 2854. DOI: 10.3389/fimmu.2018.02854. [34] MIELI-VERGANI G, VERGANI D, CZAJA AJ, et al. Autoimmune hepatitis[J]. Nat Rev Dis Primers, 2018, 4: 18017. DOI: 10.1038/nrdp.2018.17. -



 PDF下载 ( 6136 KB)
PDF下载 ( 6136 KB)

 下载:
下载: