改良白蛋白-胆红素分级对经导管动脉化疗栓塞术联合免疫及抗血管生成药物治疗的Child-Pugh A级不可切除肝细胞癌患者预后的预测价值
DOI: 10.12449/JCH241215
Value of modified albumin-bilirubin grade in predicting the prognosis of patients with Child-Pugh class A unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization combined with immunotherapy and anti-angiogenic drugs
-
摘要:
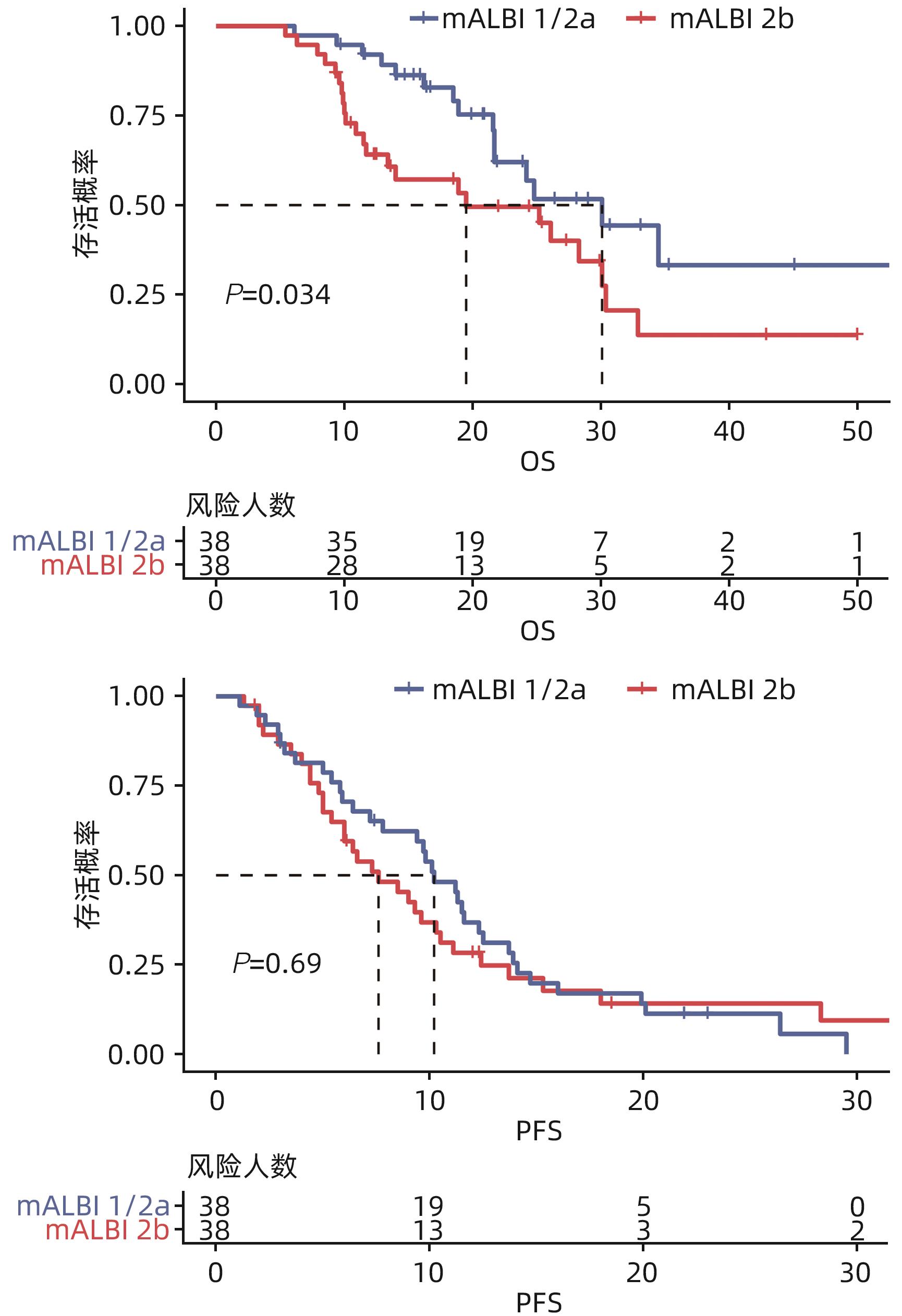
目的 研究改良白蛋白-胆红素分级(mALBI分级)对经导管动脉化疗栓塞术(TACE)联合免疫及抗血管生成药物治疗(以下简称靶免治疗)的Child-Pugh A级的不可切除肝细胞癌患者预后的评估价值。 方法 回顾性分析2020年1月—2023年1月在苏州大学附属第一医院和温州医科大学附属第五医院符合纳排标准的76例接受TACE联合靶免治疗的Child-Pugh A级不可切除肝细胞癌患者的资料,根据mALBI分级将其分为mALBI 1/2a组(n=38)和2b组(n=38)。主要研究终点为总生存期(OS),次要研究结局为无进展生存期(PFS)、客观缓解率(ORR)、疾病控制率(DCR)。评估标准包括完全缓解、部分缓解、疾病稳定以及疾病进展。符合正态分布的计量资料组间比较采用成组t检验,不符合正态分布的计量资料组间比较采用Wilcoxon秩和检验。计数资料两组间比较采用χ2检验。Kaplan-Meier法绘制生存曲线,Log-rank检验比较组间中位生存期(mOS)和中位无进展生存期(mPFS)。采用单因素和多因素Cox比例风险模型分析影响患者预后的因素。 结果 2组患者的Alb水平、肿瘤负荷情况比较,差异均有统计学意义(P值均<0.05)。76例患者的mOS为25.2(95%CI:18.4~32.0)个月,mPFS为9.4(95%CI:7.1~11.7)个月,ORR为63.2%,DCR为82.9%。其中mALBI 1/2a组和2b组患者mOS分别为30.1(95%CI:19.8~40.4)个月和19.5(95%CI:7.1~31.9)个月,两组mOS差异有统计学意义(χ2=4.490,P=0.034)。mALBI 1/2a组和2b组患者mPFS分别为10.2(95%CI:8.4~12.0)个月和7.6(95%CI:4.6~10.6)个月,ORR分别为71.1%和55.3%,DCR分别为86.8%和78.9%,mPFS、ORR和DCR比较,差异均无统计学意义(P值均>0.05)。ECOG评分、肿瘤负荷、mALBI分级、门静脉侵犯和肝外转移是影响TACE联合靶免治疗患者mOS的独立危险因素(P值均<0.05)。没有发生与治疗相关的死亡。 结论 mALBI分级对预测TACE联合靶免治疗的Child-Pugh A级不可切除肝细胞癌患者的生存有较好的预测价值。 Abstract:Objective To investigate the ability of the modified albumin-bilirubin (mALBI) grade in predicting the prognosis of patients with Child-Pugh A unresectable hepatocellular carcinoma (uHCC) after transcatheter arterial chemoembolization (TACE) combined with immunotherapy and anti-angiogenic drugs (hereafter referred to as targeted immunotherapy). Methods A retrospective analysis was performed for the data of 76 patients with Child-Pugh A uHCC who met the inclusion criteria and underwent TACE combined with targeted immunotherapy in The First Affiliated Hospital of Soochow University from January 2020 to January 2023, and according to the mALBI grade, they were divided into mALBI 1/2a group with 38 patients and mALBI 2b group with 38 patients. The primary endpoint was overall survival (OS), and the secondary endpoints were progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). Evaluation criteria included complete remission, partial remission, stable disease, and progressive disease. The independent-samples t test was used for comparison of normally distributed continuous data between groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical variables between two groups. The Kaplan-Meier method was used to plot survival curves, and the Log-rank test was used for comparison of median OS (mOS) and median PFS (mPFS) between groups. The univariate and multivariate Cox proportional hazards models were used to analyze the influencing factors for prognosis. Results There were significant differences in albumin and tumor burden between the two groups (both P<0.05). The 76 patients had an mOS of 25.2 months (95% confidence interval [CI]: 18.4 — 32.0), an mPFS of 9.4 months (95%CI: 7.1 — 11.7), an ORR of 63.2%, and a DCR of 82.9%. The mOS was 30.1 months (95%CI: 19.8 — 40.4) in the mALBI 1/2a group and 19.5 months (95%CI: 7.1 — 31.9) in the mALBI 2b group, and there was a significant difference in mOS between the two groups (χ2=4.490, P=0.034). The mALBI 1/2a group had an mPFS of 10.2 months (95%CI: 8.4 — 12.0), an ORR of 71.1%, and a DCR of 86.8%, while the mALBI 2b group had an mPFS of 7.6 months (95%CI: 4.6 — 10.6), an ORR of 55.3%, and a DCR of 78.9%; there were no significant differences in mPFS, ORR, and DCR between the two groups (all P>0.05). ECOG status, tumor burden, mALBI grade, portal vein invasion, and extrahepatic metastasis were independent risk factors for mOS in patients undergoing TACE combined with targeted immunotherapy (all P<0.05). There were no treatment-related deaths. Conclusion The mALBI grade has a good value in predicting the survival of patients with Child-Pugh A uHCC undergoing TACE combined with targeted immunotherapy. -
Key words:
- Carcinoma, Hepatocellular /
- Chemoembolization, Therapeutic /
- mALBI Grade
-
表 1 两组患者基线临床资料的比较
Table 1. Comparison of clinical baseline data of study subjects
项目 全部(n=76) mALBI 1/2a组(n=38) mALBI 2b组(n=38) 统计值 P值 年龄(岁) 59.3±10.3 58.4±10.2 60.2±10.6 t=-0.719 0.475 性别[例(%)] χ2=0.106 0.744 男 65(85.5) 32(84.2) 33(86.8) 女 11(14.5) 6(15.8) 5(13.2) 病因[例(%)] χ2=1.056 0.304 病毒性肝炎 72(94.7) 35(92.1) 37(97.4) 其他 4(5.3) 3(7.9) 1(2.6) ECOG评分[例(%)] χ2=0.054 0.817 0分 33(43.4) 17(44.7) 16(42.1) 1分 43(56.6) 21(48.8) 22(57.9) 肝硬化[例(%)] χ2=0.350 0.554 有 62(81.6) 32(84.2) 30(78.9) 无 14(18.4) 6(15.8) 8(21.1) BCLC分期[例(%)] χ2<0.001 >0.05 B 24(31.6) 12(31.6) 12(31.6) C 52(68.4) 26(68.4) 26(68.4) 血管侵犯[例(%)] χ2=0.474 0.491 有 37(48.7) 17(44.7) 20(52.6) 无 39(51.3) 21(55.3) 18(47.4) 肝外转移[例(%)] χ2=2.921 0.087 有 25(32.9) 16(42.1) 9(23.7) 无 51(67.1) 22(28.9) 29(76.3) AFP[例(%)] χ2=1.339 0.247 ≥200 ng/mL 33(43.4) 14(36.8) 19(50.0) <200 ng/mL 43(56.6) 24(63.2) 19(50.0) 肿瘤负荷[例(%)] χ2=4.070 0.044 >7 61(80.3) 27(71.1) 34(89.5) ≤7 15(19.7) 11(28.9) 4(10.5) ALT(U/L) 31.2(20.1~48.8) 27.0(20.2~43.7) 35.6(16.9~49.9) Z=-0.675 0.449 AST(U/L) 38.1(28.8~57.2) 36.4(27.5~49.7) 41.6(28.9~57.4) Z=-0.545 0.585 PLT(×109/L) 130.5(100.0~178.0) 140.0(105.5~163.5) 120.5(94.5~182.3) Z=-0.592 0.554 WBC(×109/L) 5.0(3.8~6.6) 4.5(3.7~5.5) 5.5(3.8~7.3) Z=-1.714 0.086 Lym(×109/L) 1.1(0.8~1.4) 1.2(0.9~1.4) 1.0(0.7~1.5) Z=-0.972 0.331 Nep(×109/L) 3.2(2.2~4.3) 2.7(2.2~3.6) 3.8(2.2~5.1) Z=-1.865 0.062 Cr(μmol/L) 65.2±14.9 66.8±14.2 63.7±15.6 t=0.905 0.368 Alb(g/L) 35.9±4.9 39.8±3.0 32.1±3.1 t=11.058 <0.001 靶向治疗[例(%)] χ2=6.292 0.178 索拉非尼 19(25.0) 13(34.2) 6(15.8) 仑伐替尼 33(42.3) 17(44.7) 16(42.1) 多纳非尼 1(1.3) 0(0.0) 1(2.6) 阿帕替尼 14(17.9) 4(10.5) 10(26.3) 贝伐珠单抗 9(11.5) 4(10.5) 5(13.1) 免疫治疗[例(%)] χ2=1.556 0.459 阿替利珠单抗 4(5.1) 1(2.6) 3(7.9) 信迪利单抗 36(46.2) 20(52.6) 16(42.1) 卡瑞利珠单抗 36(46.2) 17(44.7) 19(50.0) 注:Lym,淋巴细胞;Nep,中性粒细胞。
表 2 单因素及多因素Cox回归分析
Table 2. Univariate and multivariate Cox regression analyses
特征 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 性别(男 vs 女) 0.824 0.344~1.975 0.665 年龄(≥60岁 vs <60岁) 0.588 0.308~1.121 0.107 肝硬化(有 vs 无) 1.324 0.577~3.040 0.508 病毒性肝炎(是 vs 否) 0.655 0.199~2.159 0.487 ECOG评分(1分 vs 0分) 1.969 1.002~3.869 0.049 3.045 1.428~6.492 0.004 mALBI分级(≥2a vs <2a) 1.993 1.037~3.830 0.039 2.206 1.121~4.342 0.022 BCLC分期(B vs C) 0.693 0.484~0.993 0.045 0.417 0.105~1.660 0.214 ALT(U/L) 1.003 0.998~1.008 0.276 AST(U/L) 1.002 0.997~1.008 0.402 TBil(μmol/L) 1.012 0.972~1.054 0.562 Hb(g/L) 0.992 0.980~1.004 0.180 WBC(×109/L) 1.018 0.914~1.133 0.744 PLT(×109/L) 1.001 0.996~1.005 0.749 Nep(×109/L) 1.018 0.910~1.139 0.754 Lym(×109/L) 0.814 0.434~1.528 0.522 NLR 1.057 0.982~1.137 0.143 PLR 1.003 0.999~1.006 0.169 AFP(≥200 ng/mL vs <200 ng/mL) 1.217 0.640~2.313 0.549 肿瘤负荷(>7 vs ≤7) 3.095 1.094~8.753 0.033 3.734 1.245~11.193 0.019 血管侵犯(有 vs 无) 2.231 1.161~4.289 0.016 4.170 1.446~12.020 0.008 肝外转移(有 vs 无) 2.075 1.067~4.035 0.031 3.374 1.386~8.214 0.007 注:NLR,中性粒细胞与淋巴细胞比值;PLR,血小板与淋巴细胞比值。
-
[1] ZHOU MG, WANG HD, ZENG XY, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394( 10204): 1145- 1158. DOI: 10.1016/S0140-6736(19)30427-1. [2] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [3] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [4] ZHU HD, LI HL, HUANG MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma(CHANCE001)[J]. Signal Transduct Target Ther, 2023, 8( 1): 58. DOI: 10.1038/s41392-022-01235-0. [5] SANGRO B, KUDO M, QIN S, et al. A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1[J]. Ann Oncol, 2020, 31: S202- S203. DOI: 10.1016/j.annonc.2020.04.429. [6] XIN YJ, ZHANG XY, LIU N, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma[J]. Hepatol Int, 2023, 17( 3): 753- 764. DOI: 10.1007/s12072-023-10502-3. [7] Liver Oncology Branch, China Association for the Promotion of International Exchange in Healthcare; Branch Immunology, China Association for the Promotion of International Exchange in Healthcare; Cooperative Group for Chinese Expert Consensus on Targeted Immunotherapy Combined with Local Therapy for Advanced Hepatocellular Cancer. Chinese expert consensus on targeted immunotherapy combined with local therapy for advanced hepatocellular cancer[J]. J Clin Hepatol, 2023, 39( 12): 2782- 2792. DOI: 10.3969/j.issn.1001-5256.2023.12.006.中国医疗保健国际交流促进会肝脏肿瘤学分会, 中国医疗保健国际交流促进会免疫学分会,《靶免联合局部治疗中晚期肝细胞癌中国专家共识》协作组. 靶向免疫联合局部治疗中晚期肝细胞癌中国专家共识[J]. 临床肝胆病杂志, 2023, 39( 12): 2782- 2792. DOI: 10.3969/j.issn.1001-5256.2023.12.006. [8] HIRAOKA A, KUMADA T, KUDO M, et al. Albumin-bilirubin(ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan society of hepatology: A comparison with the liver damage and Child-pugh classifications[J]. Liver Cancer, 2017, 6( 3): 204- 215. DOI: 10.1159/000452846. [9] JOHNSON PJ, BERHANE S, KAGEBAYASHI C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade[J]. J Clin Oncol, 2015, 33( 6): 550- 558. DOI: 10.1200/JCO.2014.57.9151. [10] WANG YY, ZHONG JH, SU ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma[J]. Br J Surg, 2016, 103( 6): 725- 734. DOI: 10.1002/bjs.10095. [11] WANG ZX, WANG EX, XIA DD, et al. Value of Child-Pugh score versus albumin-bilirubin grade in predicting the prognosis of unresectable hepatocellular carcinoma treated by transarterial chemoembolization[J]. J Clin Hepatol, 2020, 36( 1): 113- 117. DOI: 10.3969/j.issn.1001-5256.2020.01.025.王哲轩, 王恩鑫, 夏冬东, 等. Child-Pugh评分和ALBI分级预测经肝动脉化疗栓塞治疗不可切除肝细胞癌预后的价值比较[J]. 临床肝胆病杂志, 2020, 36( 1): 113- 117. DOI: 10.3969/j.issn.1001-5256.2020.01.025. [12] KUMADA T, TOYODA H, TADA T, et al. Changes in background liver function in patients with hepatocellular carcinoma over 30 years: Comparison of child-pugh classification and albumin bilirubin grade[J]. Liver Cancer, 2020, 9( 5): 518- 528. DOI: 10.1159/000507933. [13] SUN Y, ZHANG HH, SHENG SP, et al. Prognostic significance of albumin-bilirubin grading in patients with early hepatocellular carcinoma after radiofrequency ablation treatment[J]. J Interv Radiol, 2021, 30( 5): 502- 507. DOI: 10.3969/j.issn.1008-794X.2021.05.018.孙玉, 张洪海, 生守鹏, 等. 白蛋白-胆红素分级在早期肝细胞癌射频消融中的预后意义[J]. 介入放射学杂志, 2021, 30( 5): 502- 507. DOI: 10.3969/j.issn.1008-794X.2021.05.018. [14] LEE IC, HUNG YW, LIU CA, et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma[J]. Liver Int, 2019, 39( 9): 1704- 1712. DOI: 10.1111/liv.14194. [15] SAITO N, NISHIOFUKU H, SATO T, et al. Predictive factors of complete response to transarterial chemoembolization in intermediate stage hepatocellular carcinoma beyond up-to-7 criteria[J]. Cancers, 2023, 15( 9): 2609. DOI: 10.3390/cancers15092609. [16] HICKEY R, MOULI S, KULIK L, et al. Independent analysis of albumin-bilirubin grade in a 765-patient cohort treated with transarterial locoregional therapy for hepatocellular carcinoma[J]. J Vasc Interv Radiol, 2016, 27( 6): 795- 802. DOI: 10.1016/j.jvir.2016.03.005. [17] YU WM, HONG WQ, SUN BL, et al. Value of albumin-bilirubin grade in predicting liver function changes and prognosis of hepatocellular carcinoma patients undergoing transarterial chemoembolization: A Meta-analysis[J]. J Clin Hepatol, 2021, 37( 11): 2575- 2583. DOI: 10.3969/j.issn.1001-5256.2021.11.018.余伟明, 洪文倩, 孙丙伦, 等. 白蛋白-胆红素分级对经肝动脉化疗栓塞术治疗肝癌患者肝功能变化及其预后评估价值的Meta分析[J]. 临床肝胆病杂志, 2021, 37( 11): 2575- 2583. DOI: 10.3969/j.issn.1001-5256.2021.11.018. [18] HIRAOKA A, KUMADA T, TSUJI K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis[J]. Liver Cancer, 2019, 8( 2): 121- 129. DOI: 10.1159/000488778. [19] PINATO DJ, KANEKO T, SAEED A, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: Adjunctive role of the ALBI grade[J]. Cancers, 2020, 12( 7): 1862. DOI: 10.3390/cancers12071862. [20] KUDO M, FINN RS, CHENG AL, et al. Albumin-bilirubin grade analyses of atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma: A post hoc analysis of the Phase III IMbrave150 study[J]. Liver Cancer, 2023, 12( 5): 479- 493. DOI: 10.1159/000529996. [21] TANAKA K, TSUJI K, HIRAOKA A, et al. Usefulness of tumor marker score for predicting the prognosis of hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: A multicenter retrospective study[J]. Cancers, 2023, 15( 17): 4348. DOI: 10.3390/cancers15174348. [22] KELLEY RK, RIMASSA L, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma(COSMIC-312): A multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23( 8): 995- 1008. DOI: 10.1016/S1470-2045(22)00326-6. [23] KUDO M, GALLE PR, BRANDI G, et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: Results from REACH and REACH-2[J]. JHEP Rep, 2021, 3( 2): 100215. DOI: 10.1016/j.jhepr.2020.100215. [24] LEE PC, CHEN YT, CHAO YE, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma[J]. Liver Int, 2018, 38( 2): 321- 330. DOI: 10.1111/liv.13527. [25] KUDO M. Newly developed modified ALBI grade shows better prognostic and predictive value for hepatocellular carcinoma[J]. Liver Cancer, 2022, 11( 1): 1- 8. DOI: 10.1159/000521374. [26] Clinical Guidelines Committee of Chinese College of Interventionalists. Expert consensus on the TACE failure/refractoriness and its subsequent therapies in treating patients with hepatocellular carcinoma[J]. J Interv Radiol, 2022, 31( 11): 1039- 1044. DOI: 10.3969/j.issn.1008-794X.2022.11.001.中国医师协会介入医师分会临床诊疗指南专委会. 肝细胞癌经动脉化疗栓塞抵抗及后续治疗专家共识[J]. 介入放射学杂志, 2022, 31( 11): 1039- 1044. DOI: 10.3969/j.issn.1008-794X.2022.11.001. [27] LENCIONI R, LLOVET JM. Modified RECIST(mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30( 1): 52- 60. DOI: 10.1055/s-0030-1247132. [28] PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. [29] HSU WF, HSU SC, CHEN TH, et al. Modified albumin-bilirubin model for stratifying survival in patients with hepatocellular carcinoma receiving anticancer therapy[J]. Cancers, 2022, 14( 20): 5083. DOI: 10.3390/cancers14205083. [30] IIJIMA H, KUDO M, KUBO S, et al. Report of the 23rd nationwide follow-up survey of primary liver cancer in Japan(2014-2015)[J]. Hepatol Res, 2023, 53( 10): 895- 959. DOI: 10.1111/hepr.13953. [31] AMIOKA K, KAWAOKA T, KINAMI T, et al. Analysis of lenvatinib’s efficacy against intermediate-stage unresectable hepatocellular carcinoma[J]. Cancers, 2022, 14( 20): 5066. DOI: 10.3390/cancers14205066. [32] REDDY KR, MCLERRAN D, MARSH T, et al. Incidence and risk factors for hepatocellular carcinoma in cirrhosis: The multicenter hepatocellular carcinoma early detection strategy(HEDS) study[J]. Gastroenterology, 2023, 165( 4): 1053- 1063. DOI: 10.1053/j.gastro.2023.06.027. [33] REIG M, FORNER A, RIMOLA J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update[J]. J Hepatol, 2022, 76( 3): 681- 693. DOI: 10.1016/j.jhep.2021.11.018. -



 PDF下载 ( 856 KB)
PDF下载 ( 856 KB)

 下载:
下载:


