载药微球经导管动脉化疗栓塞术联合肠系膜上动脉灌注化疗治疗肝细胞癌合并门静脉癌栓的效果分析
DOI: 10.12449/JCH241216
Efficacy of drug-eluting beads-transarterial chemoembolization combined with infusion chemotherapy via superior mesenteric artery in treatment of hepatocellular carcinoma complicated by portal vein tumor thrombus
-
摘要:
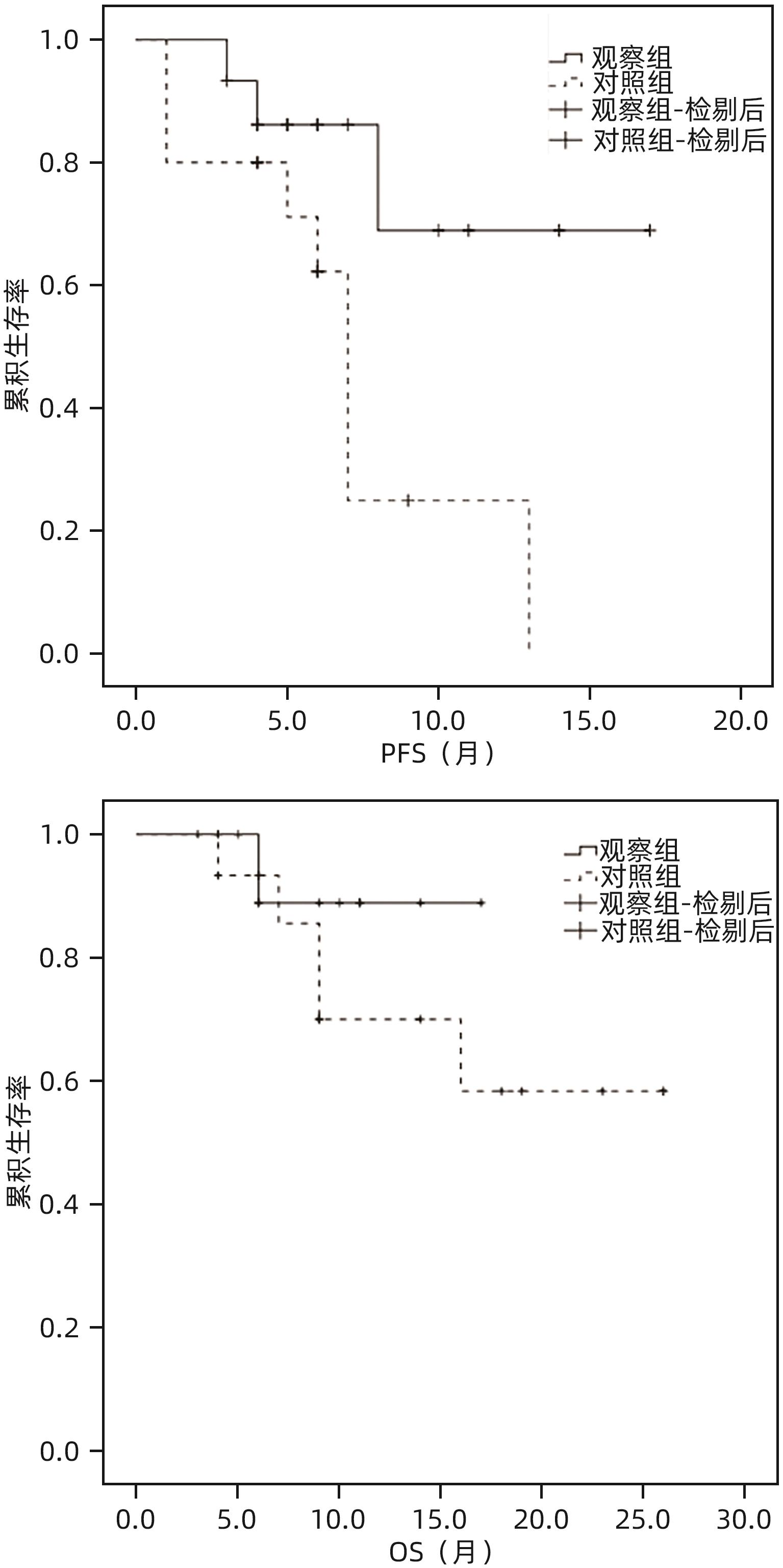
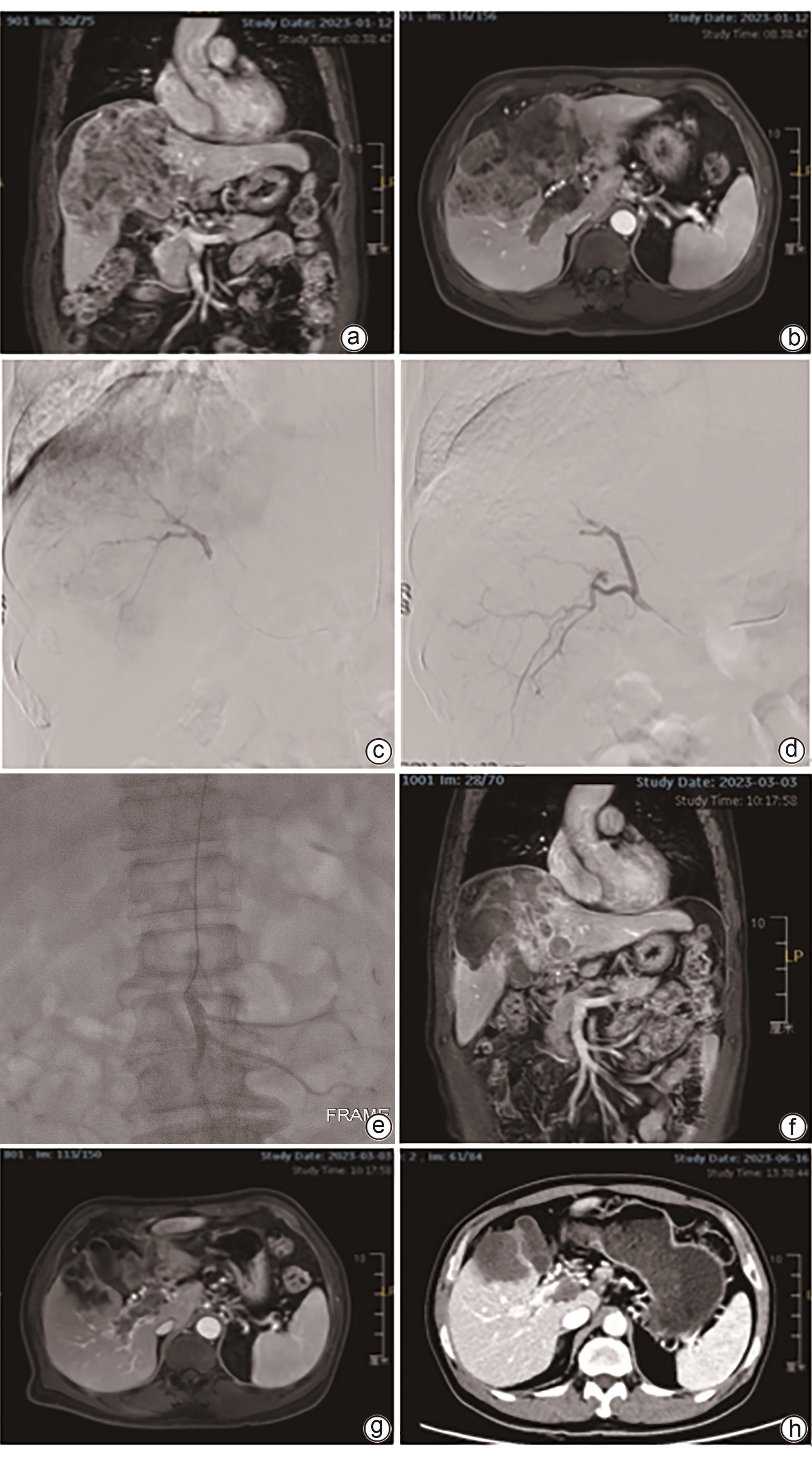
目的 对比分析载药微球经导管动脉化疗栓塞术(D-TACE)联合肠系膜上动脉灌注化疗与单纯D-TACE治疗肝细胞癌合并门静脉癌栓(PVTT)的效果。 方法 回顾性分析徐州医科大学附属医院2022年1月—2023年12月行介入治疗的肝细胞癌合并PVTT的患者资料,其中采用D-TACE联合肠系膜上动脉灌注化疗的患者15例(观察组)。按1∶1进行倾向性匹配后入选单纯D-TACE治疗的患者15例(对照组)。术后1、2、3个月及以后每3个月行上腹部增强MRI评估肝脏肿瘤及PVTT情况。对比分析两组患者客观缓解率(ORR)、疾病控制率(DCR)。计量资料两组间比较采用成组t检验或Mann-Whitney U检验,术前、术后资料比较采用配对t检验或Wilcoxon检验。计数资料两组间比较采用χ2检验。采用Kaplan-Meier曲线计算累积生存率,并使用Log-rank检验比较两组差异。 结果 两组患者治疗技术成功率100%,术后均无严重并发症。全部患者随访3~26个月,平均(10.5±6.7)个月。术后3个月,观察组和对照组肝脏肿瘤的ORR(73.3% vs 53.3%)和DCR(93.3% vs 80.0%)差异均无统计学意义(P值均>0.05);观察组PVTT的ORR(46.7% vs 13.3%)和DCR(100% vs 73.3%)均显著高于对照组(χ2值分别为3.968、4.615,P值分别为0.046、0.032)。观察组和对照组患者累积3、6、12个月无进展生存率分别为93.3%、86.2%、68.9%和80.0%、62.2%、24.9%(P=0.028);累积3、6、12个月总生存率分别为100%、88.9%、88.9%和93.3%、85.6%、70.0%(P=0.340)。 结论 与单纯D-TACE相比,D-TACE联合肠系膜上动脉灌注化疗在治疗肝细胞癌合并PVTT方面显示了更好的近期疗效。 Abstract:Objective To investigate the efficacy of drug-eluting beads-transarterial chemoembolization (D-TACE) combined with infusion chemotherapy via superior mesenteric artery versus D-TACE alone in the treatment of hepatocellular carcinoma (HCC) complicated by portal vein tumor thrombus (PVTT). Methods A retrospective analysis was performed for the data of patients with HCC and PVTT who underwent interventional treatment in The Affiliated Hospital of Xuzhou Medical University from January 2022 to December 2023, among whom 15 patients received D-TACE combined with infusion chemotherapy via superior mesenteric artery and were enrolled as observation group, and after propensity score matching at a ratio of 1∶1, 15 patients who received D-TACE alone were enrolled as control group. Contrast-enhanced MRI of the upper abdomen was performed at 1, 2, and 3 months after surgery and every 3 months thereafter to evaluate the conditions of liver tumor and PVTT. Objective response rate (ORR) and disease control rate (DCR) were compared between the two groups. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the paired t-test or the Wilcoxon test was used for comparison of preoperative and postoperative data; the chi-square test was used for comparison of categorical data between two groups. The Kaplan-Meier curve was used to calculate the cumulative survival rate, and the Log-rank test was used for comparison between two groups. Results Both groups had a technical success rate of 100%, with no serious complications after surgery. The patients were followed up for 3-26 months (mean 10.5±6.7 months). At 3 months after surgery, there were no significant differences between the observation group and the control group in ORR (73.3% vs 53.3%, χ2=1.292, P=0.256) and DCR (93.3% vs 80.0%, χ2=1.154, P=0.283) for liver tumors, and compared with the control group, the observation group had significantly higher ORR and DCR for PVTT (ORR: 46.7% vs 13.3%, χ2=3.968, P=0.046; DCR: 100% vs 73.3%, χ2=4.615, P=0.032). The 3-, 6-, and 12-month cumulative progression-free survival rates were 93.3%, 86.2%, and 68.9%, respectively, for the observation group and were 80.0%, 62.2%, and 24.9%, respectively, for the control group (P=0.028), and the 3-, 6-, and 12-month cumulative overall survival rates were 100%, 88.9%, and 88.9%, respectively, for the observation group and were 93.3%, 85.6%, and 70.0%, respectively, for the control group (P=0.340). Conclusion Compared with D-TACE alone, D-TACE combined with infusion chemotherapy via the superior mesenteric artery shows better short-term efficacy in the treatment of HCC complicated by PVTT. -
表 1 两组患者基线资料情况比较
Table 1. Comparison of baseline characteristics between the two groups of patients
指标 观察组(n=15) 对照组(n=15) 统计值 P值 年龄(岁) 58.5±10.7 57.7±8.9 t=0.315 0.755 性别[例(%)] χ2=0.370 0.543 男 14(93.3) 13(86.7) 女 1(6.7) 2(13.3) Child-Pugh评分(分) 6.3±0.9 6.1±1.1 t=0.374 0.711 Child-Pugh分级[例(%)] χ2=0.144 0.705 A 9(60.0) 10(66.7) B 6(40.0) 5(33.3) 肿瘤最大径(mm) 101.5±44.9 95.8±35.6 t=0.323 0.750 肿瘤数量[例(%)] χ2=0.536 0.464 单个 7(46.7) 9(60.0) 多个 8(53.3) 6(40.0) PVTT分型[(%)] χ2=0.311 0.958 Ⅰ型 2(13.3) 3(20.0) Ⅱ型 7(46.7) 7(46.7) Ⅲ型 5(33.3) 4(26.7) Ⅳ型 1(6.7) 1(6.7) AFP[例(%)] χ2=1.222 0.269 <400 ng/mL 7(46.7) 10(66.7) ≥400 ng/mL 8(53.3) 5(33.3) 表 2 两组患者术后1个月肝功能指标变化情况
Table 2. Changes in liver function indicators one month after treatment in the two groups of patients
指标 观察组(n=15) 对照组(n=15) 统计值 P值 AST(U/L) 术前 66.5(45.8~202.8) 61.0(33.0~117.0) Z=-0.952 0.341 术后1个月 86.0(34.0~119.0) 54.0(33.8~89.0) Z=-0.986 0.324 ALT(U/L) 术前 44.5(35.0~73.8) 42.0(22.5~80.0) Z=-0.762 0.446 术后1个月 43.5(23.3~60.3) 28.5(15.8~53.8) Z=-1.059 0.290 白蛋白(g/L) 术前 35.4±3.4 37.6±4.1 t=1.462 0.157 术后1个月 35.1±5.5 36.2±7.1 t=0.388 0.702 总胆红素(μmol/L) 术前 17.6(12.7~25.2) 14.5(11.6~23.0) Z=-0.653 0.514 术后1个月 22.5(9.9~26.7) 24.9(14.9~54.5) Z=-1.021 0.307 表 3 两组患者术后3个月疗效评价
Table 3. Assessment of efficacy at three months postoperatively in the two groups of patients
组别 例数 肝脏肿瘤[例(%)] PVTT[例(%)] 观察组 15 CR 1(6.7) 0(0.0) PR 10(66.7) 7(46.7) SD 3(20.0) 8(53.3) PD 1(6.7) 0(0.0) 对照组 15 CR 1(6.7) 0(0.0) PR 7(46.7) 2(13.3) SD 4(26.7) 9(60.0) PD 3(20.0) 4(26.7) -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] Liver Cancer Committee of Chinese Medical Doctor Association. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China(2021 edition)[J]. Natl Med J China, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn112137-20211117-02567.中国医师协会肝癌专业委员会. 中国肝细胞癌合并门静脉癌栓诊疗指南(2021年版)[J]. 中华医学杂志, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn112137-20211117-02567. [3] XIANG X, LAU WY, WU ZY, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: A multicenter study[J]. Eur J Surg Oncol, 2019, 45( 8): 1460- 1467. DOI: 10.1016/j.ejso.2019.03.042. [4] CHEN JW, LAI LS, ZHOU CR, et al. Safety, efficacy, and survival of drug-eluting beads-transarterial chemoembolization vs. conventional-transarterial chemoembolization in advanced HCC patients with main portal vein tumor thrombus[J]. Cancer Imaging, 2023, 23( 1): 70. DOI: 10.1186/s40644-023-00581-8. [5] YOON HJ, KIM JH, KIM KA, et al. Transcatheter arterial chemo-lipiodol infusion for unresectable hepatocellular carcinoma in 96 high-risk patients[J]. Clin Radiol, 2010, 65( 4): 271- 277. DOI: 10.1016/j.crad.2010.01.018. [6] LIU Y, ZHANG YW, GUO Z. Preliminary clinical experience of microparticle TACE in the treatment of hepatocellular carcinoma with portal vein tumor thrombus with rich blood supply[J]. Natl Med J China, 2014, 94( 7): 549- 550. DOI: 10.3760/cma.j.issn.0376-2491.2014.07.019.刘影, 张跃伟, 郭志. 微粒TACE治疗肝癌伴富血供门静脉癌栓的初步临床经验[J]. 中华医学杂志, 2014, 94( 7): 549- 550. DOI: 10.3760/cma.j.issn.0376-2491.2014.07.019. [7] TAN ZB, ZHANG J. Recent advances in treatment strategies for hepatocellular carcinoma with portal vein cancer thrombus[J]. Eur Rev Med Pharmacol Sci, 2023, 27( 17): 8119- 8134. DOI: 10.26355/eurrev_202309_33572. [8] Chinese Society of Liver Cancer, China Anti-Cancer Association. Chinese expert consensus on hepatic arterial infusion chemotherapy for hepatocellular carcinoma(2021 edition)[J]. Chin J Dig Surg, 2021, 20( 7): 754- 759. DOI: 10.3760/cma.j.cn115610-20210618-00288.中国抗癌协会肝癌专业委员会. 肝动脉灌注化疗治疗肝细胞癌中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20( 7): 754- 759. DOI: 10.3760/cma.j.cn115610-20210618-00288. [9] QU GL, DENG JJ, XU JX, et al.“ Dual Intervention” therapy for the primary hepatic carcinoma accompanied with portal cancerous Thrombus[J]. J Clin Radiol, 2003, 22( 8): 693- 697. DOI: 10.3969/j.issn.1001-9324.2003.08.020.屈国林, 邓京京, 徐家兴, 等. 原发性肝癌并门静脉癌栓的“双介入性”治疗[J]. 临床放射学杂志, 2003, 22( 8): 693- 697. DOI: 10.3969/j.issn.1001-9324.2003.08.020. [10] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [11] LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72( 2): 288- 306. DOI: 10.1016/j.jhep.2019.09.026. [12] LIU CF, XING WG, SI TG, et al. Efficacy and safety of apatinib combined with transarterial chemoembolization for hepatocellular carcinoma with portal venous tumor thrombus: A retrospective study[J]. Oncotarget, 2017, 8( 59): 100734- 100745. DOI: 10.18632/oncotarget.20140. [13] LU SY, YAO WY, CONG Y, et al. Retrospective analysis of 125I seed strip in the treatment of hepatocellular carcinoma with portal vein tumor thrombus and malignant obstructive jaundice[J]. Clin J Med Offic, 2024, 52( 6): 634- 637. DOI: 10.16680/j.1671-3826.2024.06.23.陆晟瑛, 姚文亿, 从云, 等. 125I粒子条治疗肝细胞癌合并门静脉癌栓伴恶性梗阻性黄疸回顾性分析[J]. 临床军医杂志, 2024, 52( 6): 634- 637. DOI: 10.16680/j.1671-3826.2024.06.23. [14] PENG LH, CHEN T, XU YXX, et al. Efficacy of mFOLFOX7 regimen systemic chemotherapy combined with camrelizumab and apatinib for hepatocellular carcinoma with Vp4 portal vain tumor thrombus[J]. Chin J Dig Surg, 2024, 23( 2): 265- 271. DOI: 10.3760/cma.j.cn115610-20240126-00044.彭林辉, 陈涛, 徐云修修, 等. mFOLFOX7方案全身化疗联合卡瑞利珠单克隆抗体和阿帕替尼治疗肝细胞癌合并Vp4型门静脉癌栓的疗效[J]. 中华消化外科杂志, 2024, 23( 2): 265- 271. DOI: 10.3760/cma.j.cn115610-20240126-00044. [15] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69( 1): 182- 236. DOI: 10.1016/j.jhep.2018.03.019. [16] REIG M, FORNER A, RIMOLA J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update[J]. J Hepatol, 2022, 76( 3): 681- 693. DOI: 10.1016/j.jhep.2021.11.018. [17] KIM GA, SHIM JH, YOON SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score analysis[J]. J Vasc Interv Radiol, 2015, 26( 3): 320- 329. e 6. DOI: 10.1016/j.jvir.2014.10.019. [18] ZHANG Y, WU JL, LI LQ. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus[J]. Ann Hepatol, 2022, 27( 1): 100552. DOI: 10.1016/j.aohep.2021.100552. [19] YANG ZW, ZOU RH, ZHENG Y, et al. Lipiodol deposition in portal vein tumour thrombus predicts treatment outcome in HCC patients after transarterial chemoembolisation[J]. Eur Radiol, 2019, 29( 11): 5752- 5762. DOI: 10.1007/s00330-019-06157-0. [20] WAN ZY, FENG GS, LIANG HM, et al. Microvascular structure and blood supply of portal vein tumor thrombus in rabbits with transplanted liver neoplasms[J]. Chin J Med Imag Technol, 2005, 21( 2): 187- 190. DOI: 10.3321/j.issn: 1003-3289.2005.02.008.万智勇, 冯敢生, 梁惠民, 等. 兔移植性肝癌门静脉癌栓的微血管结构与血供[J]. 中国医学影像技术, 2005, 21( 2): 187- 190. DOI: 10.3321/j.issn:1003-3289.2005.02.008. [21] CHOI JH, CHUNG WJ, BAE SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis[J]. Cancer Chemother Pharmacol, 2018, 82( 3): 469- 478. DOI: 10.1007/s00280-018-3638-0. [22] HE MK, LI QJ, ZOU RH, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial[J]. JAMA Oncol, 2019, 5( 7): 953- 960. DOI: 10.1001/jamaoncol.2019.0250. [23] TIAN JL, GUO SL, DU FH, et al. Pharmacokinetic comparison of intra-indirect-portal and intraportal infusion of 5-fluorouracil[J]. Chin J Hosp Pharm, 2002, 22( 7): 399- 401. DOI: 10.3321/j.issn:1001-5213.2002.07.007.田锦林, 郭顺林, 杜富会, 等. 间接门静脉与直接门静脉灌注5-氟尿嘧啶的药动学比较[J]. 中国医院药学杂志, 2002, 22( 7): 399- 401. DOI: 10.3321/j.issn:1001-5213.2002.07.007. [24] TIAN H, XU H, WANG SX, et al. Interventional chemoembolization through hepatic artery and superior mesenteric artery for primary hepatocellular carcinoma: A control study of 21 cases[J]. J Interv Radiol, 2014, 23( 8): 721- 724. DOI: 10.3969/j.issn.1008-794X.2014.08.017.田浩, 徐浩, 王诗学, 等. 经肝动脉、肠系膜上动脉双途径治疗原发性肝癌21例对照研究[J]. 介入放射学杂志, 2014, 23( 8): 721- 724. DOI: 10.3969/j.issn.1008-794X.2014.08.017. [25] DAI WC, ZANG MY, YUAN GS, et al. Efficacy of hepatic arterial infusion chemotherapy and its multimodality therapeutic regimens in treatment of patients with advanced hepatocellular carcinoma and related prognostic factors[J]. J Clin Hepatol, 2023, 39( 7): 1592- 1599. DOI: 10.3969/j.issn.1001-5256.2023.07.013.戴文聪, 臧梦雅, 袁国盛, 等. 肝动脉灌注化疗及其综合治疗方案对中晚期肝细胞癌患者的临床疗效及预后因素分析[J]. 临床肝胆病杂志, 2023, 39( 7): 1592- 1599. DOI: 10.3969/j.issn.1001-5256.2023.07.013. -



 PDF下载 ( 1735 KB)
PDF下载 ( 1735 KB)

 下载:
下载: