外周时钟基因在非酒精性脂肪性肝炎发展与防治中的作用
DOI: 10.12449/JCH241222
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:刘思言负责设计论文框架,查找文献,起草论文;田静怡负责查找文献,绘制图表及文章撰写;黄雨扬、谷天麒、邓茗月负责文章撰写和修改;杨攀负责拟定写作思路,指导撰写文章并最后定稿。
Role of peripheral clock genes in the progression, prevention, and treatment of nonalcoholic steatohepatitis
-
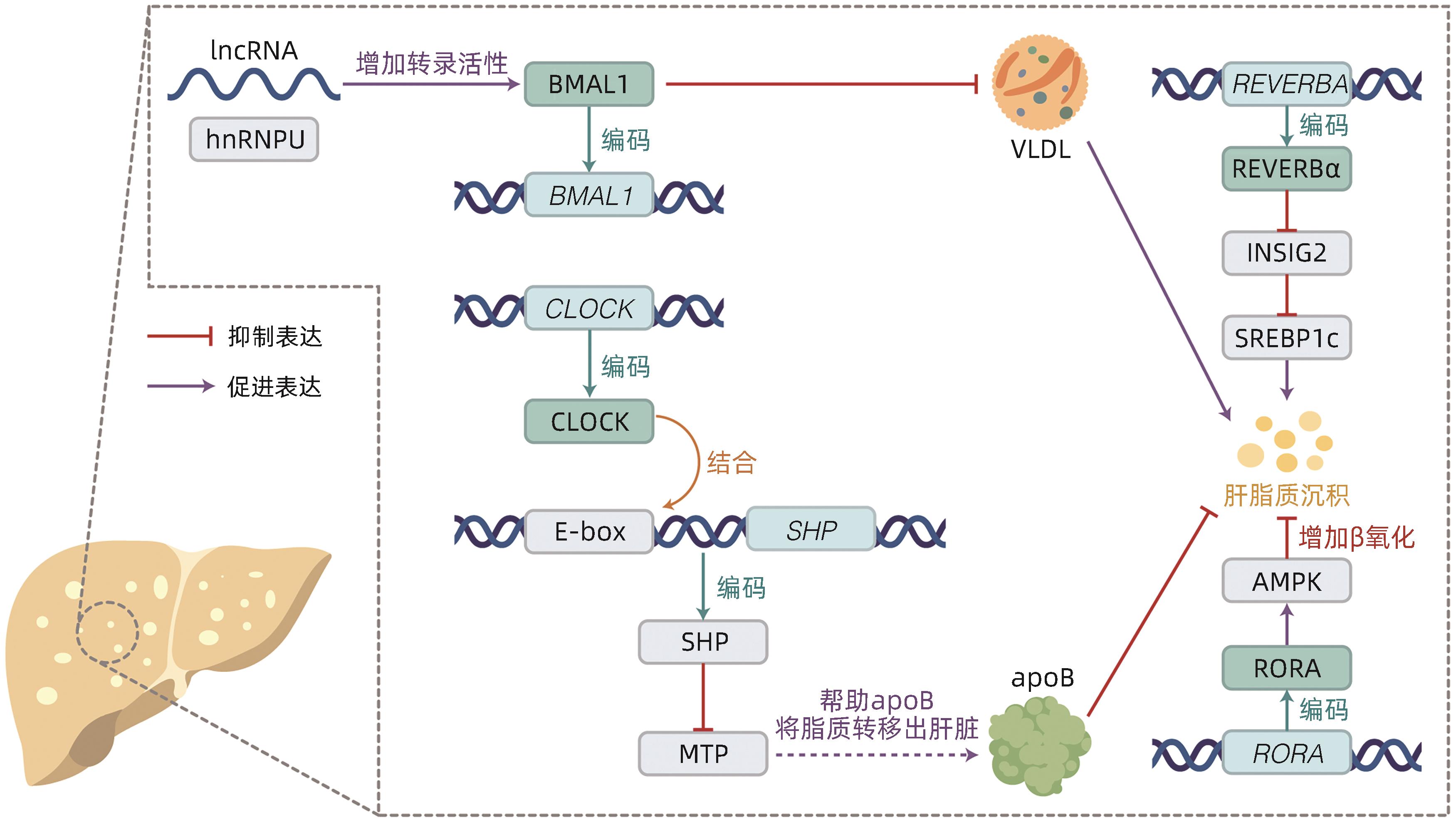
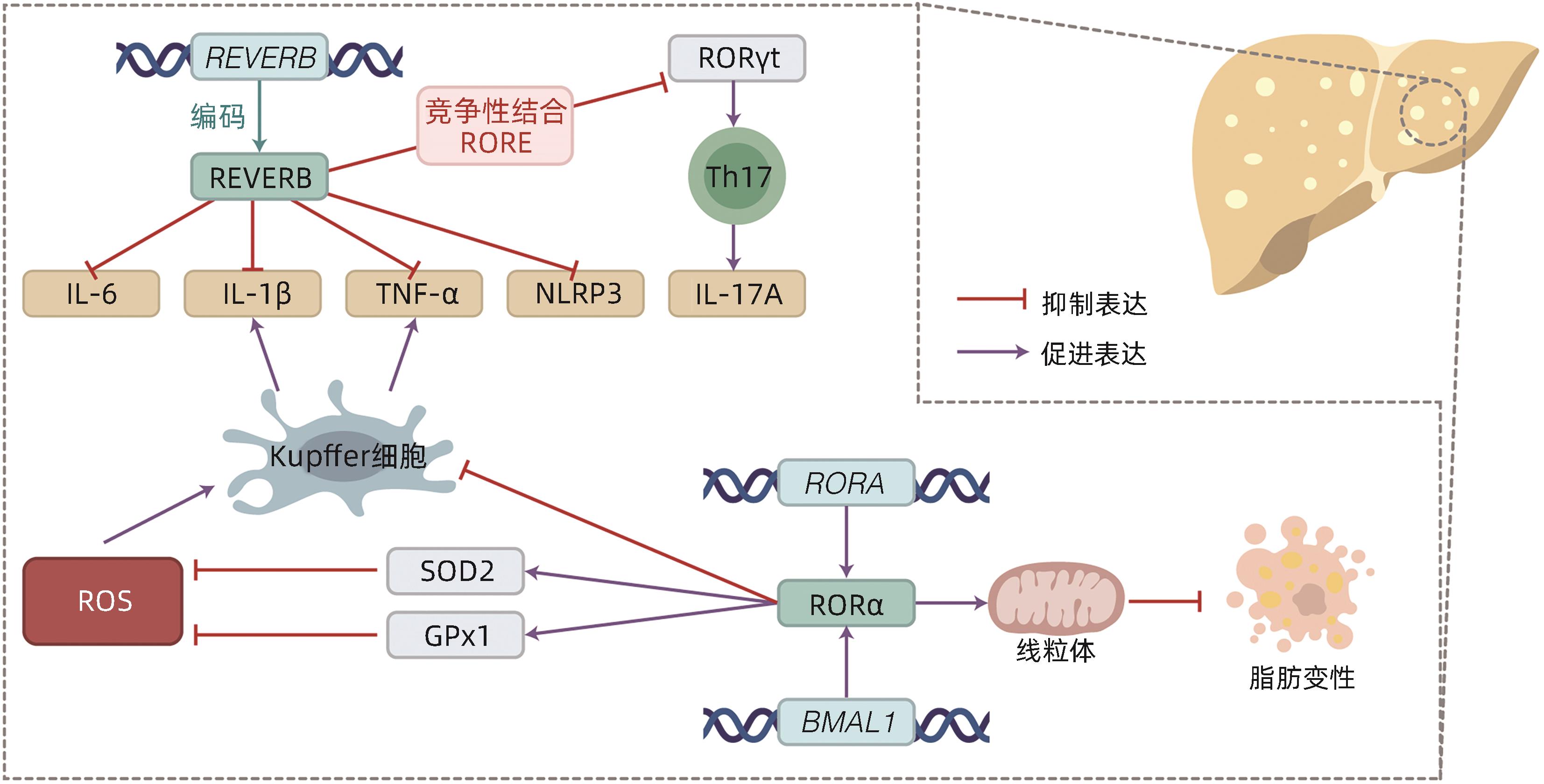
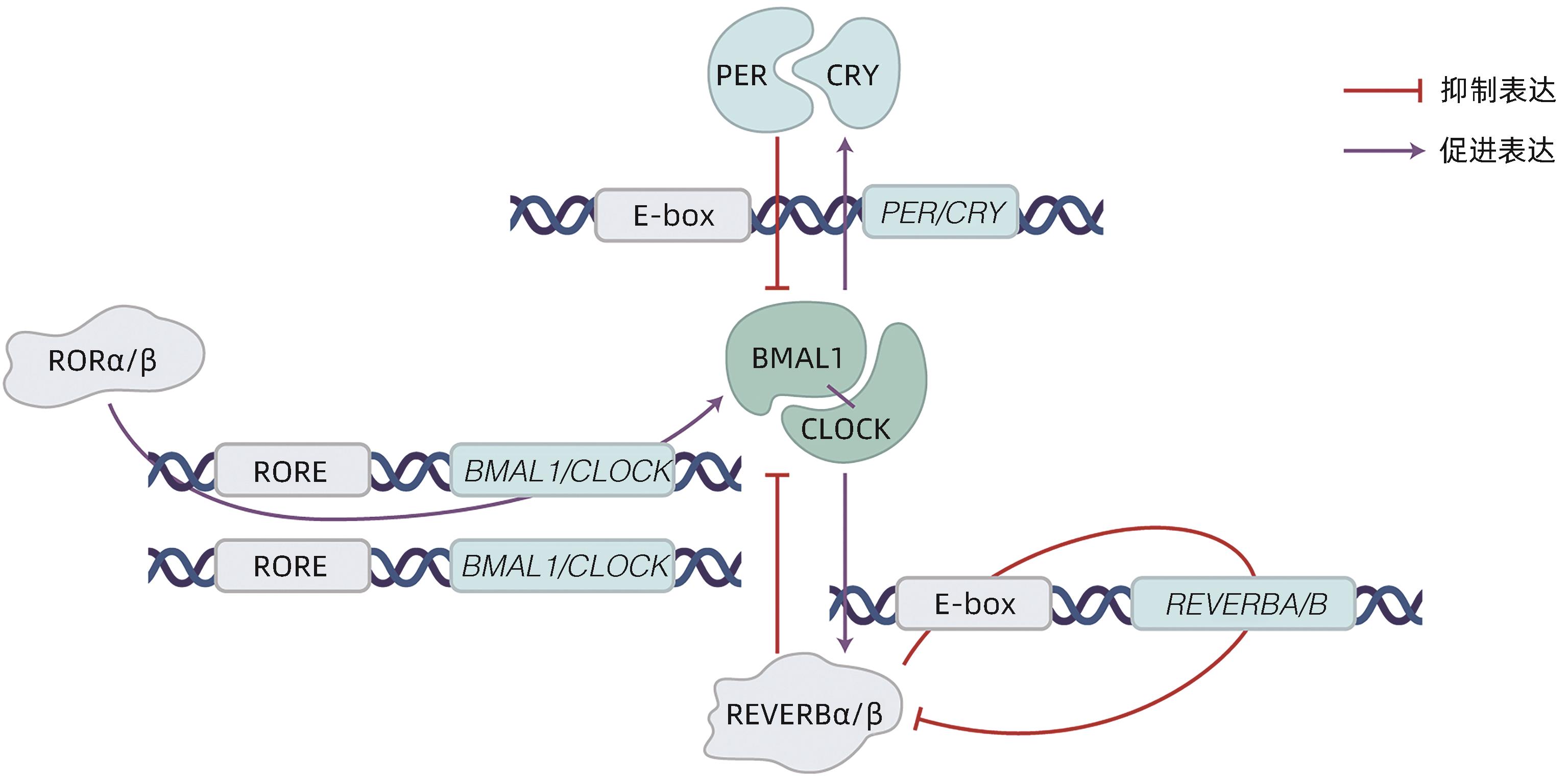
摘要: 非酒精性脂肪性肝炎(NASH)作为非酒精性脂肪性肝病的严重临床表现形式,以肝脂质沉积、炎症损伤为特征。目前NASH的临床治疗药物仍处于探索实验阶段,亟待取得进展。有研究指出NASH的发病机制与肝脏昼夜节律紊乱有关,具体可表现为肝时钟基因如BMAL1等表达失调,导致肝内脂质生成增多、脂肪酸氧化减少以及促炎症因子激活。因此,改善肝脏昼夜节律,调节肝时钟基因表达被视为防治NASH的可行策略。目前已有通过激活时钟基因编码蛋白治疗NASH的药物应用于动物实验,如REVERB全激动剂SR9009,其能抑制肝脏的炎症发展,有效证实了靶向时钟编码蛋白在NASH治疗中的可能性。本文总结了肝时钟基因在调节肝脂质代谢与炎症发生发展中的作用,阐述了近年来以时钟基因及其相关蛋白作为靶点的药物研究进展,以期为NASH治疗提供新靶点。Abstract: As a severe clinical manifestation of nonalcoholic fatty liver disease, nonalcoholic steatohepatitis (NASH) is characterized by lipid deposition and inflammatory damage in the liver. At present, clinical medications for NASH are still in the exploratory phase, and it is urgent to make progress. Recent studies have shown that the pathogenesis of NASH is associated with circadian rhythm disorders in the liver, with the specific manifestation of dysregulated expression of liver clock genes such as BMAL1, which increases hepatic lipogenesis, reduces fatty acid oxidation, and activates pro-inflammatory factors. Therefore, improving circadian rhythm of the liver and regulating the expression of liver clock genes are feasible strategies for the prevention and treatment of NASH. Currently, some medications for NASH via activating the proteins encoded by clock genes have been applied in animal experiments, for example, the REVERB full-agonist SR9009 can inhibit the development of liver inflammation, which confirms the possibility of NASH treatment by targeting the proteins encoded by clock genes. This article summarizes the role of hepatic clock genes in regulating lipid metabolism and the development and progression of inflammation in the liver and elaborates on the recent advances in medications targeting clock genes and the proteins encoded by clock genes, in order to provide new targets for the treatment of NASH.
-
Key words:
- Non-alcoholic Fatty Liver Disease /
- Circadian Rhythm /
- CLOCK Proteins
-
-
[1] HUANG DQ, EL-SERAG HB, LOOMBA R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 4): 223- 238. DOI: 10.1038/s41575-020-00381-6. [2] GRANDER C, GRABHERR F, TILG H. Non-alcoholic fatty liver disease: Pathophysiological concepts and treatment options[J]. Cardiovasc Res, 2023, 119( 9): 1787- 1798. DOI: 10.1093/cvr/cvad095. [3] ZOU HM, GE Y, LEI Q, et al. Epidemiology and disease burden of non-alcoholic steatohepatitis in greater China: A systematic review[J]. Hepatol Int, 2022, 16( 1): 27- 37. DOI: 10.1007/s12072-021-10286-4. [4] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [5] DUFOUR JF, ANSTEE QM, BUGIANESI E, et al. Current therapies and new developments in NASH[J]. Gut, 2022, 71( 10): 2123- 2134. DOI: 10.1136/gutjnl-2021-326874. [6] GREENWELL BJ, TROTT AJ, BEYTEBIERE JR, et al. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice[J]. Cell Rep, 2019, 27( 3): 649- 657. DOI: 10.1016/j.celrep.2019.03.064. [7] KIM HJ, HAN YH, NA H, et al. Liver-specific deletion of RORα aggravates diet-induced nonalcoholic steatohepatitis by inducing mitochondrial dysfunction[J]. Sci Rep, 2017, 7( 1): 16041. DOI: 10.1038/s41598-017-16077-y. [8] FAGIANI F, MARINO DD, ROMAGNOLI A, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases[J]. Signal Transduct Target Ther, 2022, 7( 1): 41. DOI: 10.1038/s41392-022-00899-y. [9] VANDENBERGHE A, LEFRANC M, FURLAN A. An overview of the circadian clock in the frame of chronotherapy: From bench to bedside[J]. Pharmaceutics, 2022, 14( 7): 1424. DOI: 10.3390/pharmaceutics14071424. [10] MUKHERJI A, BAILEY SM, STAELS B, et al. The circadian clock and liver function in health and disease[J]. J Hepatol, 2019, 71( 1): 200- 211. DOI: 10.1016/j.jhep.2019.03.020. [11] ABE YO, YOSHITANE H, KIM DW, et al. Rhythmic transcription of Bmal1 stabilizes the circadian timekeeping system in mammals[J]. Nat Commun, 2022, 13( 1): 4652. DOI: 10.1038/s41467-022-32326-9. [12] SHEN X, ZHANG Y, JI X, et al. Long noncoding RNA lncRHL regulates hepatic VLDL secretion by modulating hnRNPU/BMAL1/MTTP axis[J]. Diabetes, 2022, 71( 9): 1915- 1928. DOI: 10.2337/db21-1145. [13] YE CS, ZHANG YJ, LIN SM, et al. Berberine ameliorates metabolic-associated fatty liver disease mediated metabolism disorder and redox homeostasis by upregulating clock genes: Clock and Bmal1 expressions[J]. Molecules, 2023, 28( 4): 1874. DOI: 10.3390/molecules28041874. [14] LIN HG, WANG L, LIU ZH, et al. Hepatic MDM2 causes metabolic associated fatty liver disease by blocking triglyceride-VLDL secretion via ApoB degradation[J]. Adv Sci(Weinh), 2022, 9( 20): e2200742. DOI: 10.1002/advs.202200742. [15] PAN XY, ZHANG YX, WANG L, et al. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP[J]. Cell Metab, 2010, 12( 2): 174- 186. DOI: 10.1016/j.cmet.2010.05.014. [16] BOLSHETTE N, IBRAHIM H, REINKE H, et al. Circadian regulation of liver function: From molecular mechanisms to disease pathophysiology[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 11): 695- 707. DOI: 10.1038/s41575-023-00792-1. [17] REINKE H, ASHER G. Crosstalk between metabolism and circadian clocks[J]. Nat Rev Mol Cell Biol, 2019, 20( 4): 227- 241. DOI: 10.1038/s41580-018-0096-9. [18] GRIFFETT K, HAYES ME, BOECKMAN MP, et al. The role of REV-ERB in NASH[J]. Acta Pharmacol Sin, 2022, 43( 5): 1133- 1140. DOI: 10.1038/s41401-022-00883-w. [19] LEE KC, WU PS, LIN HC. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis[J]. Clin Mol Hepatol, 2023, 29( 1): 77- 98. DOI: 10.3350/cmh.2022.0237. [20] CHHUNCHHA B, KUBO ER, SINGH DP. Clock protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6[J]. Cells, 2020, 9( 8): 1861. DOI: 10.3390/cells9081861. [21] LENNICKE C, COCHEMÉ HM. Redox regulation of the insulin signalling pathway[J]. Redox Biol, 2021, 42: 101964. DOI: 10.1016/j.redox.2021.101964. [22] WANG S, LIN YK, YUAN X, et al. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis[J]. Nat Commun, 2018, 9( 1): 4246. DOI: 10.1038/s41467-018-06568-5. [23] HAN YH, KIM HJ, NA H, et al. RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis[J]. Cell Rep, 2017, 20( 1): 124- 135. DOI: 10.1016/j.celrep.2017.06.017. [24] KIM HJ, HAN YH, KIM JY, et al. RORα enhances lysosomal acidification and autophagic flux in the hepatocytes[J]. Hepatol Commun, 2021, 5( 12): 2121- 2138. DOI: 10.1002/hep4.1785. [25] GUAN DY, BAE H, ZHOU DS, et al. Hepatocyte SREBP signaling mediates clock communication within the liver[J]. J Clin Invest, 2023, 133( 8): e163018. DOI: 10.1172/JCI163018. [26] NI YH, ZHAO YF, MA LY, et al. Pharmacological activation of REV-ERBα improves nonalcoholic steatohepatitis by regulating intestinal permeability[J]. Metabolism, 2021, 114: 154409. DOI: 10.1016/j.metabol.2020.154409. [27] LEE YS, CHA BY, SAITO K, et al. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice[J]. Biochem Pharmacol, 2010, 79( 11): 1674- 1683. DOI: 10.1016/j.bcp.2010.01.034. [28] KHAMBU B, YAN SM, HUDA N, et al. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease[J]. Liver Res, 2018, 2( 3): 112- 119. DOI: 10.1016/j.livres.2018.09.004. [29] SARAN AR, DAVE S, ZARRINPAR A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease[J]. Gastroenterology, 2020, 158( 7): 1948- 1966. DOI: 10.1053/j.gastro.2020.01.050. [30] FANG CQ, PAN JH, QU N, et al. The AMPK pathway in fatty liver disease[J]. Front Physiol, 2022, 13: 970292. DOI: 10.3389/fphys.2022.970292. [31] SHI DM, CHEN J, WANG JF, et al. Circadian clock genes in the metabolism of non-alcoholic fatty liver disease[J]. Front Physiol, 2019, 10: 423. DOI: 10.3389/fphys.2019.00423. [32] ZHAO HK, WU L, YAN GF, et al. Inflammation and tumor progression: Signaling pathways and targeted intervention[J]. Signal Transduct Target Ther, 2021, 6( 1): 263. DOI: 10.1038/s41392-021-00658-5. [33] SINGH V, UBAID S. Role of silent information regulator 1(SIRT1) in regulating oxidative stress and inflammation[J]. Inflammation, 2020, 43( 5): 1589- 1598. DOI: 10.1007/s10753-020-01242-9. [34] HAN SC, LI ZZ, HAN F, et al. ROR alpha protects against LPS-induced inflammation by down-regulating SIRT1/NF-kappa B pathway[J]. Arch Biochem Biophys, 2019, 668: 1- 8. DOI: 10.1016/j.abb.2019.05.003. [35] CHYAU CC, WANG HF, ZHANG WJ, et al. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway[J]. Int J Mol Sci, 2020, 21( 1): 360. DOI: 10.3390/ijms21010360. [36] ZHANG CY, TAN XH, YANG HH, et al. COX-2/sEH dual inhibitor alleviates hepatocyte senescence in NAFLD mice by restoring autophagy through Sirt1/PI3K/AKT/mTOR[J]. Int J Mol Sci, 2022, 23( 15): 8267. DOI: 10.3390/ijms23158267. [37] ZHOU B, ZHANG Y, ZHANG F, et al. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1[J]. Hepatology, 2014, 59( 6): 2196- 2206. DOI: 10.1002/hep.26992. [38] ZHONG DD, CAI J, HU C, et al. Inhibition of mPGES-2 ameliorates NASH by activating NR1D1 via heme[J]. Hepatology, 2023, 78( 2): 547- 561. DOI: 10.1002/hep.32671. [39] SUN Y, JIA ZJ, YANG GR, et al. mPGES-2 deletion remarkably enhances liver injury in streptozotocin-treated mice via induction of GLUT2[J]. J Hepatol, 2014, 61( 6): 1328- 1336. DOI: 10.1016/j.jhep.2014.07.018. [40] LI RB, XIN T, LI DD, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy[J]. Redox Biol, 2018, 18: 229- 243. DOI: 10.1016/j.redox.2018.07.011. [41] WU SN, LU QL, WANG QL, et al. Binding of FUN14 domain containing 1 with inositol 1, 4, 5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo[J]. Circulation, 2017, 136( 23): 2248- 2266. DOI: 10.1161/CIRCULATIONAHA.117.030235. [42] CUI AY, DING D, LI Y. Regulation of hepatic metabolism and cell growth by the ATF/CREB family of transcription factors[J]. Diabetes, 2021, 70( 3): 653- 664. DOI: 10.2337/dbi20-0006. [43] SHIMIZU-ALBERGINE M, BASU D, KANTER JE, et al. CREBH normalizes dyslipidemia and halts atherosclerosis in diabetes by decreasing circulating remnant lipoproteins[J]. J Clin Invest, 2021, 131( 22): e153285. DOI: 10.1172/JCI153285. [44] YANG Z, KIM H, ALI A, et al. Interaction between stress responses and circadian metabolism in metabolic disease[J]. Liver Res, 2017, 1( 3): 156- 162. DOI: 10.1016/j.livres.2017.11.002. -



 PDF下载 ( 1717 KB)
PDF下载 ( 1717 KB)

 下载:
下载: