双硫死亡在非酒精性脂肪性肝病中的作用机制
DOI: 10.12449/JCH241223
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:严丽沙、陈煜负责查阅文献,撰写论文;严丽沙、孙洁、冯宪敏负责资料分析,设计课题;陈煜、王学士参与论文撰写及修改;孙洁和冯宪敏指导论文撰写、修改及校阅。
-
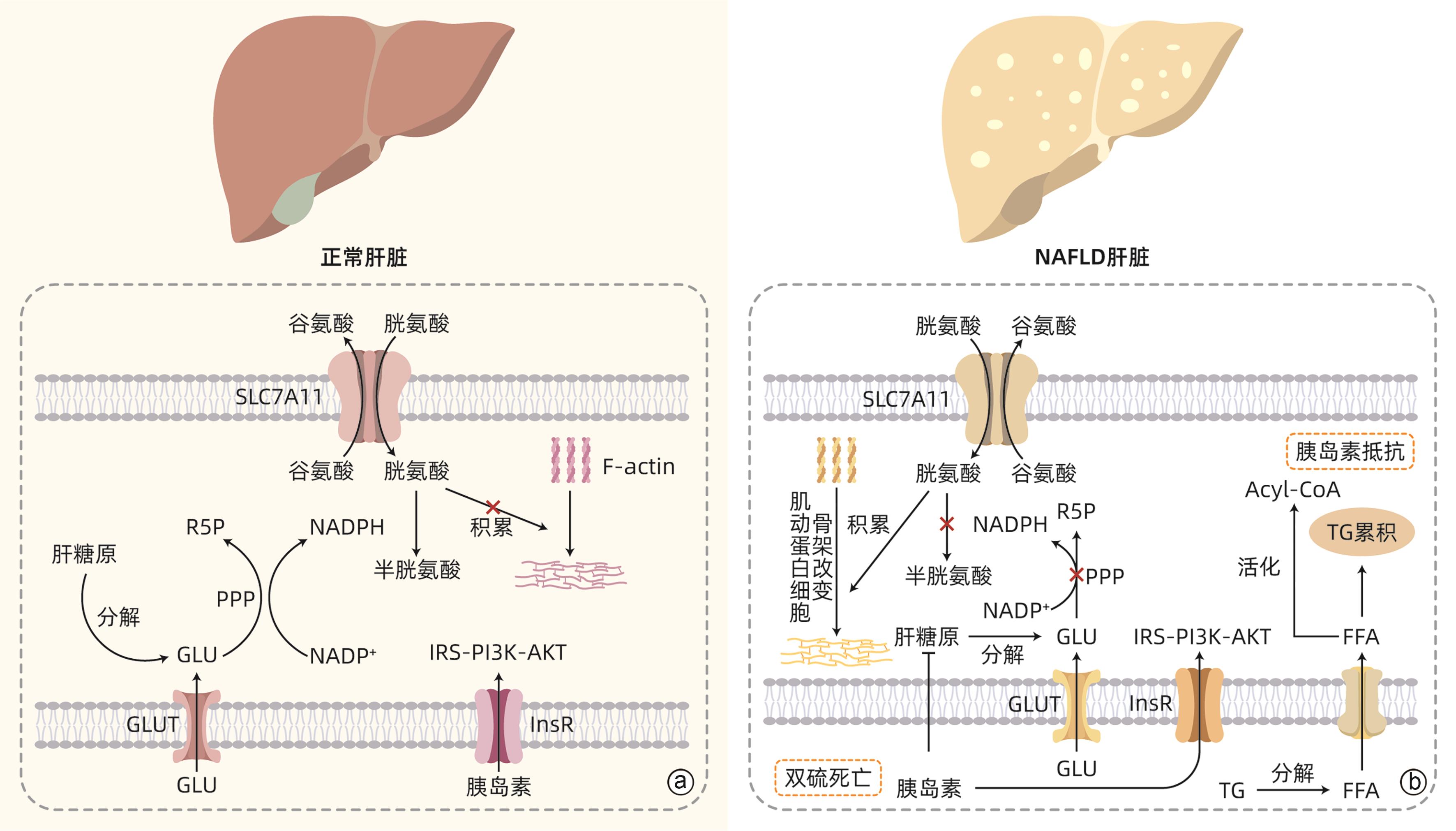
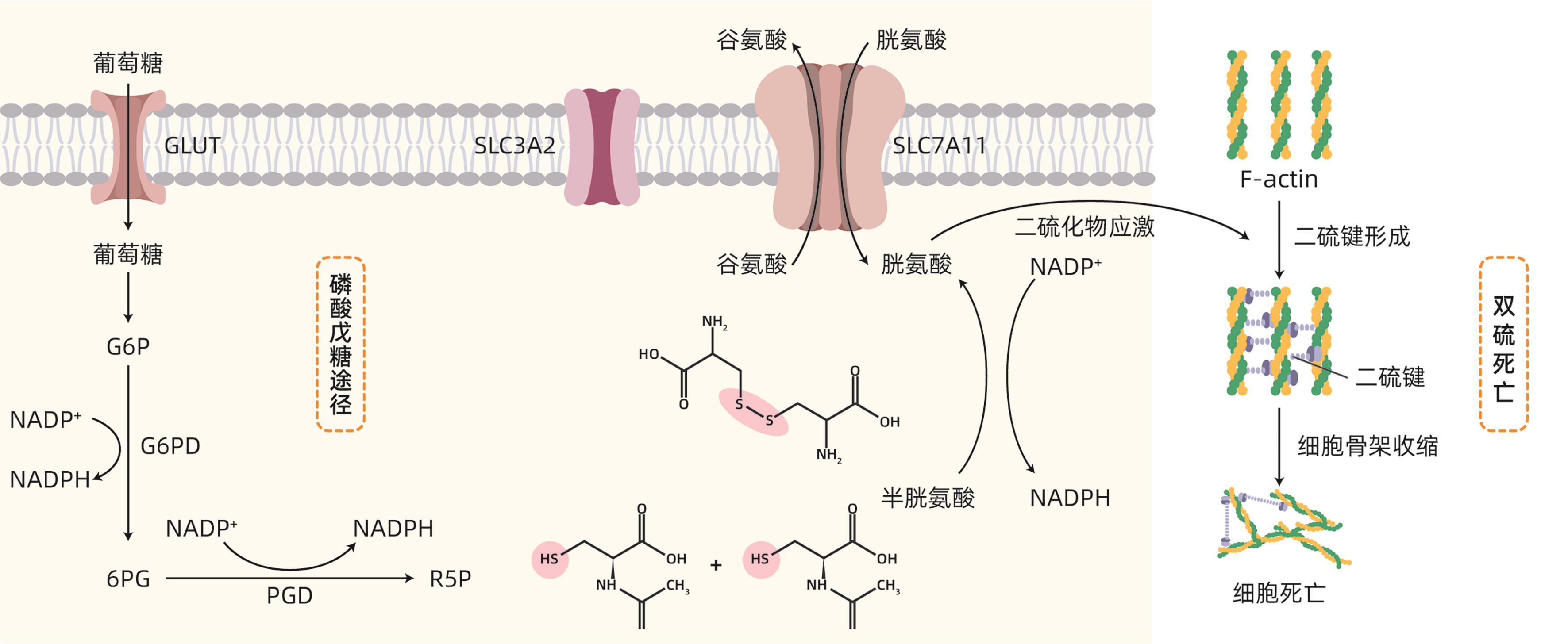
摘要: 双硫死亡是近年来提出的一种新型细胞死亡方式,其本质为烟酰胺腺嘌呤二核苷酸磷酸不足而导致的二硫化物应激性死亡。非酒精性脂肪性肝病(NAFLD)是一类以脂肪浸润为主要病理特征,与胰岛素抵抗和遗传易感性密切相关的代谢性疾病。最新研究显示,双硫死亡产生的二硫化物应激可导致肝细胞死亡,从而加快NAFLD的进展。本文就双硫死亡在NAFLD中的最新研究予以总结和分析,以期探讨双硫死亡在NAFLD中的应用潜力,为NAFLD的防治提供新思路。Abstract: Disulfidptosis is a novel pattern of cell death caused by disulfide stress and inadequate NADPH. Nonalcoholic fatty liver disease (NAFLD) is a group of metabolic diseases with the main pathological feature of fatty infiltration, and it is closely associated with insulin resistance and genetic susceptibility. Currently, the latest studies have shown that disulfide stress caused by disulfidptosis can result in hepatocyte death, thereby accelerating the progression of NAFLD. This article summarizes and analyzes the latest studies on disulfidptosis in NAFLD, in order to explore the application of disulfidptosis in NAFLD and provide new ideas for the prevention and treatment of NAFLD.
-
Key words:
- Non-alcoholic Fatty Liver Disease /
- Disulfidptosis /
- Pathologic Processes
-
-
[1] AMORIM R, SOARES P, CHAVARRIA D, et al. Decreasing the burden of non-alcoholic fatty liver disease: From therapeutic targets to drug discovery opportunities[J]. Eur J Med Chem, 2024, 277: 116723. DOI: 10.1016/j.ejmech.2024.116723. [2] YANG BM, TANG GM, WANG MT, et al. Trimethylamine N-oxide induces non-alcoholic fatty liver disease by activating the PERK[J]. Toxicol Lett, 2024, 400: 93- 103. DOI: 10.1016/j.toxlet.2024.08.009. [3] YOUNOSSI ZM, GOLABI P, PAIK JM, et al. The global epidemiology of nonalcoholic fatty liver disease(NAFLD) and nonalcoholic steatohepatitis(NASH): A systematic review[J]. Hepatology, 2023, 77( 4): 1335- 1347. DOI: 10.1097/HEP.0000000000000004. [4] LUO XH, GUO JJ, DENG HB, et al. Unveiling the role of disulfidptosis-related genes in the pathogenesis of non-alcoholic fatty liver disease[J]. Front Immunol, 2024, 15: 1386905. DOI: 10.3389/fimmu.2024.1386905. [5] LIU XG, NIE LT, ZHANG YL, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis[J]. Nat Cell Biol, 2023, 25( 3): 404- 414. DOI: 10.1038/s41556-023-01091-2. [6] ZHENG TJ, LIU QB, XING FY, et al. Disulfidptosis: A new form of programmed cell death[J]. J Exp Clin Cancer Res, 2023, 42( 1): 137. DOI: 10.1186/s13046-023-02712-2. [7] SARMIENTO-SALINAS FL, PEREZ-GONZALEZ A, ACOSTA-CASIQUE A, et al. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression[J]. Life Sci, 2021, 284: 119942. DOI: 10.1016/j.lfs.2021.119942. [8] NJEIM R, ALKHANSA S, FORNONI A. Unraveling the crosstalk between lipids and NADPH oxidases in diabetic kidney disease[J]. Pharmaceutics, 2023, 15( 5): 1360. DOI: 10.3390/pharmaceutics15051360. [9] MUSAOGULLARI A, CHAI YC. Redox regulation by protein S-glutathionylation: From molecular mechanisms to implications in health and disease[J]. Int J Mol Sci, 2020, 21( 21): 8113. DOI: 10.3390/ijms21218113. [10] SHUAI Y, MA ZH, YUAN P. Disulfidptosis: Disulfide stress-induced novel cell death pathway[J]. MedComm(2020), 2024, 5( 7): e579. DOI: 10.1002/mco2.579. [11] YUE JD, YIN YK, FENG XJ, et al. Discovery of the inhibitor targeting the SLC7A11/xCT axis through in silico and in vitro experiments[J]. Int J Mol Sci, 2024, 25( 15): 8284. DOI: 10.3390/ijms25158284. [12] MACHESKY LM. Deadly actin collapse by disulfidptosis[J]. Nat Cell Biol, 2023, 25( 3): 375- 376. DOI: 10.1038/s41556-023-01100-4. [13] SHUBHRASMITA SAHU S, SARKAR P, CHATTOPADHYAY A. Quantitation of F-actin in cytoskeletal reorganization: Context, methodology and implications[J]. Methods, 2024, 230: 44- 58. DOI: 10.1016/j.ymeth.2024.07.009. [14] YANG GN, KOPECKI Z, COWIN AJ. Role of actin cytoskeleton in the regulation of epithelial cutaneous stem cells[J]. Stem Cells Dev, 2016, 25( 10): 749- 759. DOI: 10.1089/scd.2016.0051. [15] DEWANE G, SALVI AM, DEMALI KA. Fueling the cytoskeleton-links between cell metabolism and actin remodeling[J]. J Cell Sci, 2021, 134( 3): jcs248385. DOI: 10.1242/jcs.248385. [16] JYOTSANA N, TA KT, DELGIORNO KE. The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of cancer[J]. Front Oncol, 2022, 12: 858462. DOI: 10.3389/fonc.2022.858462. [17] XU ZC, WANG YP, YANG WL, et al. Total extracts from Abelmoschus manihot(L.) alleviate radiation-induced cardiomyocyte ferroptosis via regulating redox imbalances mediated by the NOX4/xCT/GPX4 axis[J]. J Ethnopharmacol, 2024, 334: 118582. DOI: 10.1016/j.jep.2024.118582. [18] COSTA I, BARBOSA DJ, BENFEITO S, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases[J]. Pharmacol Ther, 2023, 244: 108373. DOI: 10.1016/j.pharmthera.2023.108373. [19] KOPPULA P, ZHUANG L, GAN BY. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy[J]. Protein Cell, 2021, 12( 8): 599- 620. DOI: 10.1007/s13238-020-00789-5. [20] MURRAY TV, DONG X, SAWYER GJ, et al. NADPH oxidase 4 regulates homocysteine metabolism and protects against acetaminophen-induced liver damage in mice[J]. Free Radic Biol Med, 2015, 89: 918- 930. DOI: 10.1016/j.freeradbiomed.2015.09.015. [21] COLLET JF, CHO SH, IORGA BI, et al. How the assembly and protection of the bacterial cell envelope depend on cysteine residues[J]. J Biol Chem, 2020, 295( 34): 11984- 11994. DOI: 10.1074/jbc.REV120.011201. [22] ADEVA-ANDANY MM, PÉREZ-FELPETE N, FERNÁNDEZ-FERNÁNDEZ C, et al. Liver glucose metabolism in humans[J]. Biosci Rep, 2016, 36( 6): e00416. DOI: 10.1042/BSR20160385. [23] GARCÍA-DOMÍNGUEZ E, CARRETERO A, VIÑA-ALMUNIA A, et al. Glucose 6-P dehydrogenase-an antioxidant enzyme with regulatory functions in skeletal muscle during exercise[J]. Cells, 2022, 11( 19): 3041. DOI: 10.3390/cells11193041. [24] SONG WX, LI DY, TAO L, et al. Solute carrier transporters: The metabolic gatekeepers of immune cells[J]. Acta Pharm Sin B, 2020, 10( 1): 61- 78. DOI: 10.1016/j.apsb.2019.12.006. [25] YU XX, GUO ZH, FANG ZH, et al. Identification and validation of disulfidptosis-associated molecular clusters in non-alcoholic fatty liver disease[J]. Front Genet, 2023, 14: 1251999. DOI: 10.3389/fgene.2023.1251999. [26] AHMED B, SULTANA R, GREENE MW. Adipose tissue and insulin resistance in obese[J]. Biomed Pharmacother, 2021, 137: 111315. DOI: 10.1016/j.biopha.2021.111315. [27] AMINI-SALEHI E, LETAFATKAR N, NOROUZI N, et al. Global prevalence of nonalcoholic fatty liver disease: An updated review meta-analysis comprising a population of 78 million from 38 countries[J]. Arch Med Res, 2024, 55( 6): 103043. DOI: 10.1016/j.arcmed.2024.103043. [28] OH AR, JEONG Y, YU JJ, et al. Hepatocyte Kctd17 inhibition ameliorates glucose intolerance and hepatic steatosis caused by obesity-induced chrebp stabilization[J]. Gastroenterology, 2023, 164( 3): 439- 453. DOI: 10.1053/j.gastro.2022.11.019. [29] ATORRASAGASTI C, ONORATO AM, MAZZOLINI G. The role of SPARC(secreted protein acidic and rich in cysteine) in the pathogenesis of obesity, type 2 diabetes, and non-alcoholic fatty liver disease[J]. J Physiol Biochem, 2023, 79( 4): 815- 831. DOI: 10.1007/s13105-022-00913-5. [30] AJOOLABADY A, KAPLOWITZ N, LEBEAUPIN C, et al. Endoplasmic reticulum stress in liver diseases[J]. Hepatology, 2023, 77( 2): 619- 639. DOI: 10.1002/hep.32562. [31] CELIK C, LEE SYT, YAP WS, et al. Endoplasmic reticulum stress and lipids in health and diseases[J]. Prog Lipid Res, 2023, 89: 101198. DOI: 10.1016/j.plipres.2022.101198. [32] LIANG YC, KAUSHAL D, WILSON RB. Cellular senescence and extracellular vesicles in the pathogenesis and treatment of obesity-A narrative review[J]. Int J Mol Sci, 2024, 25( 14): 7943. DOI: 10.3390/ijms25147943. [33] de ALMEIDA CHUFFA LG, SEIVA FRF, SILVEIRA HS, et al. Melatonin regulates endoplasmic reticulum stress in diverse pathophysiological contexts: A comprehensive mechanistic review[J]. J Cell Physiol, 2024: e31383. DOI: 10.1002/jcp.31383. [34] ZHANG J, GUO JF, YANG NN, et al. Endoplasmic reticulum stress-mediated cell death in liver injury[J]. Cell Death Dis, 2022, 13( 12): 1051. DOI: 10.1038/s41419-022-05444-x. [35] SIMÕES ICM, AMORIM R, TEIXEIRA J, et al. The alterations of mitochondrial function during NAFLD progression-an independent effect of mitochondrial ROS production[J]. Int J Mol Sci, 2021, 22( 13): 6848. DOI: 10.3390/ijms22136848. [36] KARKUCINSKA-WIECKOWSKA A, SIMOES ICM, KALINOWSKI P, et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship[J]. Eur J Clin Invest, 2022, 52( 3): e13622. DOI: 10.1111/eci.13622. [37] LIU XG, ZHUANG L, GAN BY. Disulfidptosis: Disulfide stress-induced cell death[J]. Trends Cell Biol, 2024, 34( 4): 327- 337. DOI: 10.1016/j.tcb.2023.07.009. [38] JOLY JH, DELFARAH A, PHUNG PS, et al. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose[J]. J Biol Chem, 2020, 295( 5): 1350- 1365. DOI: 10.1074/jbc.RA119.011471. [39] HORNA-TERRÓN E, PRADILLA-DIESTE A, SÁNCHEZ-DE-DIEGO C, et al. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology[J]. Int J Mol Sci, 2014, 15( 12): 23501- 23518. DOI: 10.3390/ijms151223501. [40] NATH B, SZABO G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases[J]. Hepatology, 2012, 55( 2): 622- 633. DOI: 10.1002/hep.25497. [41] GONG H, HE QD, ZHU LL, et al. Associations between systemic inflammation indicators and nonalcoholic fatty liver disease: Evidence from a prospective study[J]. Front Immunol, 2024, 15: 1389967. DOI: 10.3389/fimmu.2024.1389967. [42] LIU Q, BENGMARK S, QU S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease(NAFLD)[J]. Lipids Health Dis, 2010, 9: 42. DOI: 10.1186/1476-511X-9-42. [43] BURGER D, FICKENTSCHER C, de MOERLOOSE P, et al. F-actin dampens NLRP3 inflammasome activity via Flightless-I and LRRFIP2[J]. Sci Rep, 2016, 6: 29834. DOI: 10.1038/srep29834. [44] de CARVALHO RIBEIRO M, SZABO G. Role of the inflammasome in liver disease[J]. Annu Rev Pathol, 2022, 17: 345- 365. DOI: 10.1146/annurev-pathmechdis-032521-102529. [45] YU LL, HONG W, LU S, et al. The NLRP3 inflammasome in non-alcoholic fatty liver disease and steatohepatitis: Therapeutic targets and treatment[J]. Front Pharmacol, 2022, 13: 780496. DOI: 10.3389/fphar.2022.780496. [46] LEE PP, LOBATO-MÁRQUEZ D, PRAMANIK N, et al. Wiskott-Aldrich syndrome protein regulates autophagy and inflammasome activity in innate immune cells[J]. Nat Commun, 2017, 8( 1): 1576. DOI: 10.1038/s41467-017-01676-0. [47] ELMORSY EA, SABER S, HAMAD RS, et al. Modulating the HSP90 control over NFκB/NLRP3/caspase-1 axis is a new therapeutic target in the management of liver fibrosis: Insights into the role of TAS-116(pimitespib)[J]. Life Sci, 2024, 354: 122966. DOI: 10.1016/j.lfs.2024.122966. [48] RAMOS-TOVAR E, MURIEL P. NLRP3 inflammasome in hepatic diseases: A pharmacological target[J]. Biochem Pharmacol, 2023, 217: 115861. DOI: 10.1016/j.bcp.2023.115861. [49] SATHEESAN A, KUMAR J, LEELA KV, et al. Review on the role of nucleotide-binding oligomerization domain-like receptor protein 3(NLRP3) inflammasome pathway in diabetes: Mechanistic insights and therapeutic implications[J]. Inflammopharmacology, 2024, 32( 5): 2753- 2779. DOI: 10.1007/s10787-024-01556-2. [50] BARROW ER, VALIONYTE E, BAXTER CR, et al. Discovery of SQSTM1/p62-dependent P-bodies that regulate the NLRP3 inflammasome[J]. Cell Rep, 2024, 43( 3): 113935. DOI: 10.1016/j.celrep.2024.113935. -



 PDF下载 ( 1236 KB)
PDF下载 ( 1236 KB)

 下载:
下载: