药物性肝损伤的免疫学机制
DOI: 10.12449/JCH241227
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:王宇负责课题设计,拟定写作思路,撰写论文;刘成海、李爽指导撰写文章并最后定稿。
-
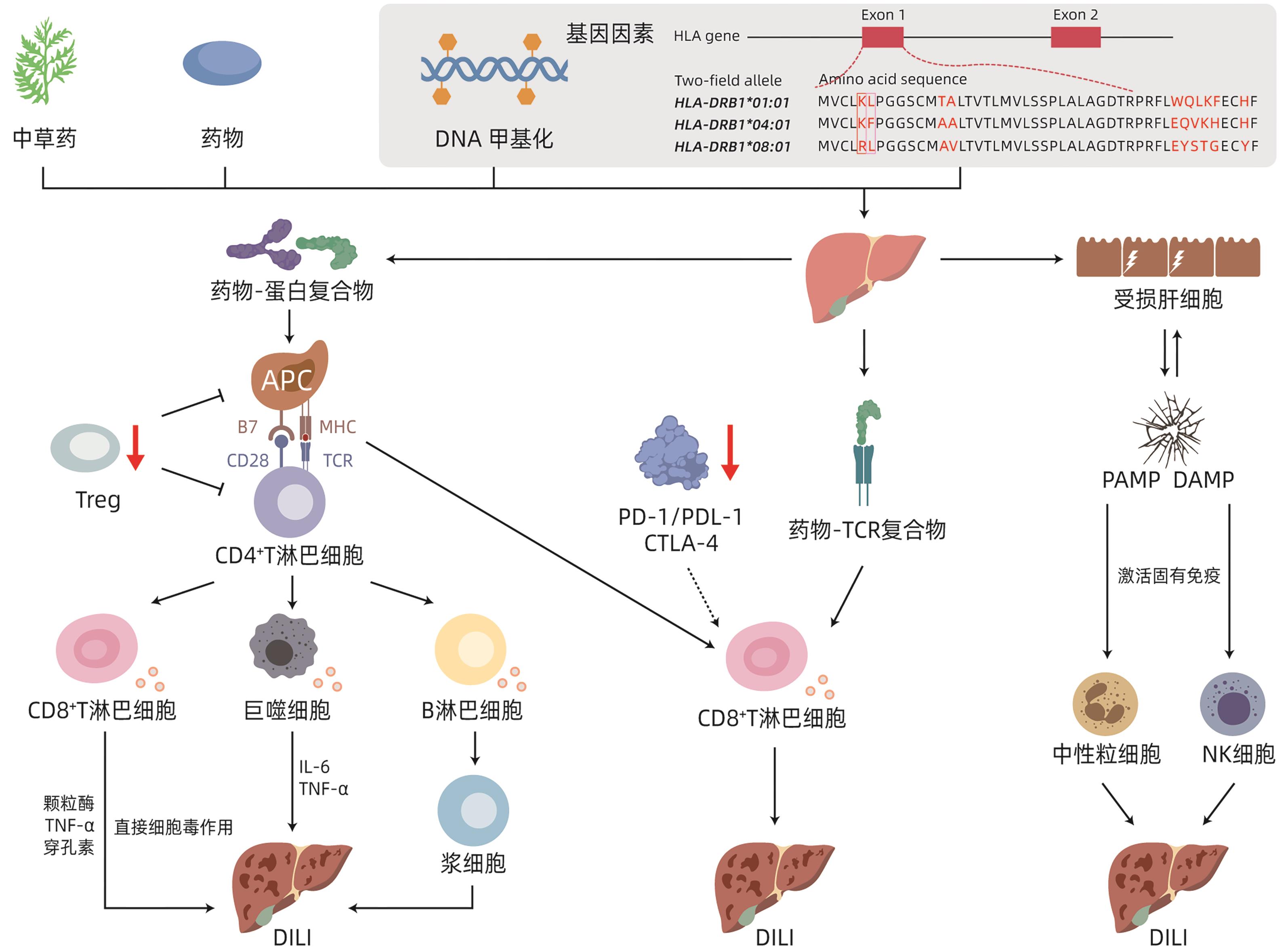
摘要: 药物性肝损伤(DILI)机制复杂,涉及多种途径协同促进并互为因果,其中免疫反应在致病机制中扮演重要角色。在遗传背景因素下,药物活性代谢产物、药物-分子复合物、危险信号分子或可作为DILI发生的免疫反应触发因子,激活药物抗原性超敏反应机制、P-i假说和固有免疫危险信号机制。使肝脏出现固有免疫、适应性免疫级联反应,导致肝脏固有免疫耐受状态失衡,进而造成肝脏组织的免疫炎症性损伤。本文主要阐述DILI的免疫学发生机制,以期为DILI治疗靶点的研发及规避用药不良反应等提供新的思路。
-
关键词:
- 化学性与药物性肝损伤 /
- 免疫 /
- 治疗学
Abstract: Drug-induced liver injury (DILI) has a complex mechanism involving various pathways with a synergistic effect on each other and a causal relationship with each other, among which immune response plays an important role in the pathogenesis of DILI. In the context of genetic background, drug active metabolites, drug-molecule complexes, and danger signal molecules may be used as the trigger factors for immune response in DILI, activating the mechanism of drug antigenic hypersensitivity, P-i hypothesis, and danger signal mechanism in innate immunity. The cascade reaction of innate immunity and adaptive immunity leads to the imbalance of the innate immune tolerance of the liver and thus causes immunoinflammatory injury of liver tissue. This article mainly elaborates on the immunological mechanism of DILI, in order to provide new ideas for the research and development of therapeutic targets for DILI and the methods for avoiding adverse drug reactions.-
Key words:
- Chemical and Drug Induced Liver Injury /
- Immunity /
- Therapeutics
-
[1] Technology Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association; Study Group of Drug-Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases. Chinese guideline for diagnosis and management of drug-induced liver injury(2023 version)[J]. Chin J Gastroenterol, 2023, 28( 7): 397- 431. DOI: 10.3760/cma.j.cn501113-20230419-00176.中国医药生物技术协会药物性肝损伤防治技术专业委员会, 中华医学会肝病学分会药物性肝病学组. 中国药物性肝损伤诊治指南(2023年版)[J]. 胃肠病学, 2023, 28( 7): 397- 431. DOI: 10.3760/cma.j.cn501113-20230419-00176. [2] RANI J, DHULL SB, ROSE PK, et al. Drug-induced liver injury and anti-hepatotoxic effect of herbal compounds: A metabolic mechanism perspective[J]. Phytomedicine, 2024, 122: 155142. DOI: 10.1016/j.phymed.2023.155142. [3] SINGH S, KUMAR PVSNK, KUMAR JP, et al. Genetic and epigenetic basis of drug-induced liver injury[J]. Semin Liver Dis, 2023, 43( 2): 163- 175. DOI: 10.1055/a-2097-0531. [4] ZHANG D, HAO JQ, HOU RL, et al. The role of NAT2 polymorphism and methylation in anti-tuberculosis drug-induced liver injury in Mongolian tuberculosis patients[J]. J Clin Pharm Ther, 2020, 45( 3): 561- 569. DOI: 10.1111/jcpt.13097. [5] DEVARBHAVI H, PATIL M, MENON M. Association of human leukocyte antigen-B*13:01 with dapsone-induced liver injury[J]. Br J Clin Pharmacol, 2022, 88( 3): 1369- 1372. DOI: 10.1111/bcp.15054. [6] ASIF BA, KOH C, PHILLIPS EJ, et al. Vancomycin-induced liver injury, DRESS, and HLA-a 32:01[J]. J Allergy Clin Immunol Pract, 2024, 12( 1): 168- 174. DOI: 10.1016/j.jaip.2023.09.011. [7] NICOLETTI P, DELLINGER A, LI YJ, et al. Identification of reduced ERAP2 expression and a novel HLA allele as components of a risk score for susceptibility to liver injury due to amoxicillin-clavulanate[J]. Gastroenterology, 2023, 164( 3): 454- 466. DOI: 10.1053/j.gastro.2022.11.036. [8] JING J, HE TT, BAI ZF, et al. An excerpt from AASLD Practice Guidance on drug, herbal and dietary supplement-induced liver injury[J]. J Clin Hepatol, 2022, 38( 10): 2219- 2223 DOI: 10.3969/j.issn.1001-5256.2022.10.005.景婧, 何婷婷, 柏兆方, 等.《2022年美国肝病学会实践指南:药物、草药和膳食补充剂诱导的肝损伤》摘译[J]. 临床肝胆病杂志, 2022, 38( 10): 2219- 2223. DOI: 10.3969/j.issn.1001-5256.2022.10.005. [9] HOOFNAGLE JH, BONKOVSKY HL, PHILLIPS EJ, et al. HLA-B*35:01 and green tea-induced liver injury[J]. Hepatology, 2021, 73( 6): 2484- 2493. DOI: 10.1002/hep.31538. [10] SANTOS EA, GONÇALVES JCS, FLEURY MK, et al. Relationship of anti-tuberculosis drug-induced liver injury and genetic polymorphisms in CYP2E1 and GST[J]. Braz J Infect Dis, 2019, 23( 6): 381- 387. DOI: 10.1016/j.bjid.2019.09.003. [11] CHEN SX, PAN HQ, CHEN YZ, et al. Association between genetic polymorphisms of NRF2, KEAP1, MAFF, MAFK and anti-tuberculosis drug-induced liver injury: A nested case-control study[J]. Sci Rep, 2019, 9( 1): 14311. DOI: 10.1038/s41598-019-50706-y. [12] KOIDO M, KAWAKAMI E, FUKUMURA J, et al. Polygenic architecture informs potential vulnerability to drug-induced liver injury[J]. Nat Med, 2020, 26( 10): 1541- 1548. DOI: 10.1038/s41591-020-1023-0. [13] ANTOINE DJ, WILLIAMS DP, KIPAR A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo[J]. Toxicol Sci, 2009, 112( 2): 521- 531. DOI: 10.1093/toxsci/kfp235. [14] LIU ZC, WANG YP, BORLAK J, et al. Mechanistically linked serum miRNAs distinguish between drug induced and fatty liver disease of different grades[J]. Sci Rep, 2016, 6: 23709. DOI: 10.1038/srep23709. [15] FROMENTY B. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury[J]. Food Chem Toxicol, 2020, 135: 110916. DOI: 10.1016/j.fct.2019.110916. [16] CHONG YZ, ZHU HY, REN Q, et al. Interaction between the HIF-1α gene rs1957757 polymorphism and CpG island methylation in the promoter region is associated with the risk of anti-tuberculosis drug-induced liver injury in humans: A case-control study[J]. J Clin Pharm Ther, 2022, 47( 7): 948- 955. DOI: 10.1111/jcpt.13625. [17] WEI YQ, HUAI C, ZHOU CX, et al. A methylation functional detection hepatic cell system validates correlation between DNA methylation and drug-induced liver injury[J]. Pharmacogenomics J, 2020, 20( 5): 717- 723. DOI: 10.1038/s41397-020-0160-7. [18] LAMMERT C, ZHU CS, LIAN Y, et al. Exploratory study of autoantibody profiling in drug-induced liver injury with an autoimmune phenotype[J]. Hepatol Commun, 2020, 4( 11): 1651- 1663. DOI: 10.1002/hep4.1582. [19] LEE SK, CHOI JY, JUNG ES, et al. An immunological perspective on the mechanism of drug induced liver injury: Focused on drugs for treatment of hepatocellular carcinoma and liver transplantation[J]. Int J Mol Sci, 2023, 24( 5): 5002. DOI: 10.3390/ijms24055002. [20] Food and Drug Allergy Prevention Subgroup, Allergy Prevention and Control Committee of Chinese Preventive Medicine Association. Chinese expert consensus on approach to the diagnosis and prevention of drug allergy[J]. Chin J Prev Med, 2022, 56( 6): 682- 706. DOI: 10.3760/cma.j.cn112150-20220129-00100.中华预防医学会过敏病预防与控制专业委员会预防食物药物过敏学组. 药物过敏诊断和预防方案中国专家共识[J]. 中华预防医学杂志, 2022, 56( 6): 682- 706. DOI: 10.3760/cma.j.cn112150-20220129-00100. [21] TAN CK, HO D, WANG LM, et al. Drug-induced autoimmune hepatitis: A minireview[J]. World J Gastroenterol, 2022, 28( 24): 2654- 2666. DOI: 10.3748/wjg.v28.i24.2654. [22] CHEN CW. Autoimmune liver harm induced by medication[J]. Drug Eval, 2007, 4( 5): 323- 325. DOI: 10.3969/j.issn.1672-2809.2007.05.002.陈成伟. 药物诱导的自身免疫性肝损害[J]. 药品评价, 2007, 4( 5): 323- 325. DOI: 10.3969/j.issn.1672-2809.2007.05.002. [23] BJÖRNSSON HK, GUDBJORNSSON B, BJÖRNSSON ES. Infliximab-induced liver injury: Clinical phenotypes, autoimmunity and the role of corticosteroid treatment[J]. J Hepatol, 2022, 76( 1): 86- 92. DOI: 10.1016/j.jhep.2021.08.024. [24] WEBER S, BENESIC A, BUCHHOLTZ ML, et al. Antimitochondrial rather than antinuclear antibodies correlate with severe drug-induced liver injury[J]. Dig Dis, 2021, 39( 3): 275- 282. DOI: 10.1159/000511635. [25] YAN MZ, ZHAO C, LU SY, et al. Trimethylamine N-oxide exacerbates acetaminophen-induced liver injury by interfering with macrophage-mediated liver regeneration[J]. J Cell Physiol, 2022, 237( 1): 897- 910. DOI: 10.1002/jcp.30568. [26] BOETTLER T, CSERNALABICS B, SALIÉ H, et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis[J]. J Hepatol, 2022, 77( 3): 653- 659. DOI: 10.1016/j.jhep.2022.03.040. [27] MONSHI MM, FAULKNER L, GIBSON A, et al. Human leukocyte antigen(HLA)-B*57: 01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury[J]. Hepatology, 2013, 57( 2): 727- 739. DOI: 10.1002/hep.26077. [28] PUIG M, ANANTHULA S, VENNA R, et al. Alterations in the HLA-B*57:01 immunopeptidome by flucloxacillin and immunogenicity of drug-haptenated peptides[J]. Front Immunol, 2021, 11: 629399. DOI: 10.3389/fimmu.2020.629399. [29] ANANTHULA S, KRISHNAVENI SIVAKUMAR K, CARDONE M, et al. Development of mouse models with restricted HLA-B 57: 01 presentation for the study of flucloxacillin-driven T-cell activation and tolerance in liver injury[J]. J Allergy Clin Immunol, 2023, 152( 2): 486- 499. e 7. DOI: 10.1016/j.jaci.2023.03.029. [30] HERNANDEZ N, BESSONE F. Hepatotoxicity induced by biological agents: Clinical features and current controversies[J]. J Clin Transl Hepatol, 2022, 10( 3): 486- 495. DOI: 10.14218/JCTH.2021.00243. [31] FERNANDEZ-SANTAMARIA R, ARIZA A, FERNANDEZ TD, et al. Advances and highlights in T and B cell responses to drug antigens[J]. Allergy, 2022, 77( 4): 1129- 1138. DOI: 10.1111/all.15126. [32] LI DP, CHEN Y, WAN MJ, et al. Oral magnesium prevents acetaminophen-induced acute liver injury by modulating microbial metabolism[J]. Cell Host Microbe, 2024, 32( 1): 48- 62. DOI: 10.1016/j.chom.2023.11.006. [33] ZENG YN, WU R, WANG FZ, et al. Liberation of daidzein by gut microbial β-galactosidase suppresses acetaminophen-induced hepatotoxicity in mice[J]. Cell Host Microbe, 2023, 31( 5): 766- 780. DOI: 10.1016/j.chom.2023.04.002. [34] SUN MD, CHEN PY, XIAO K, et al. Circulating cell-free DNAs as a biomarker and therapeutic target for acetaminophen-induced liver injury[J]. Adv Sci, 2023, 10( 16): e2206789. DOI: 10.1002/advs.202206789. [35] ROTH RA, MAIURI AR, GANEY PE. Idiosyncratic drug-induced liver injury: Is drug-cytokine interaction the linchpin?[J]. J Pharmacol Exp Ther, 2017, 360( 2): 461- 470. DOI: 10.1124/jpet.116.237578. [36] LAI RT, XIANG XG, MO RD, et al. Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury[J]. J Hepatol, 2015, 63( 1): 148- 155. DOI: 10.1016/j.jhep.2015.02.004. [37] WANG XF, SUN R, CHEN YY, et al. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury[J]. Int Immunopharmacol, 2015, 25( 2): 293- 301. DOI: 10.1016/j.intimp.2015.02.008. [38] WANG Y, LI ZY, LI S, et al. Research advances in adverse liver reactions caused by immune checkpoint inhibitors[J]. J Clin Hepatol, 2022, 38( 1): 220- 223. DOI: 10.3969/j.issn.1001-5256.2022.01.039.王宇, 李钊颖, 李爽, 等. 免疫检查点抑制剂所致肝脏不良反应的研究进展[J]. 临床肝胆病杂志, 2022, 38( 1): 220- 223. DOI: 10.3969/j.issn.1001-5256.2022.01.039. [39] CUNNINGHAM M, GUPTA R, BUTLER M. Checkpoint inhibitor hepatotoxicity: Pathogenesis and management[J]. Hepatology, 2024, 79( 1): 198- 212. DOI: 10.1097/HEP.0000000000000045. -



 PDF下载 ( 956 KB)
PDF下载 ( 956 KB)

 下载:
下载:


