胆汁淤积性肝病的临床管理
DOI: 10.12449/JCH250702
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:郭悦承负责收集资料和撰写论文;蔡晓波负责拟定写作思路和课题设计;陆伦根负责指导撰写文章并最后定稿。
-
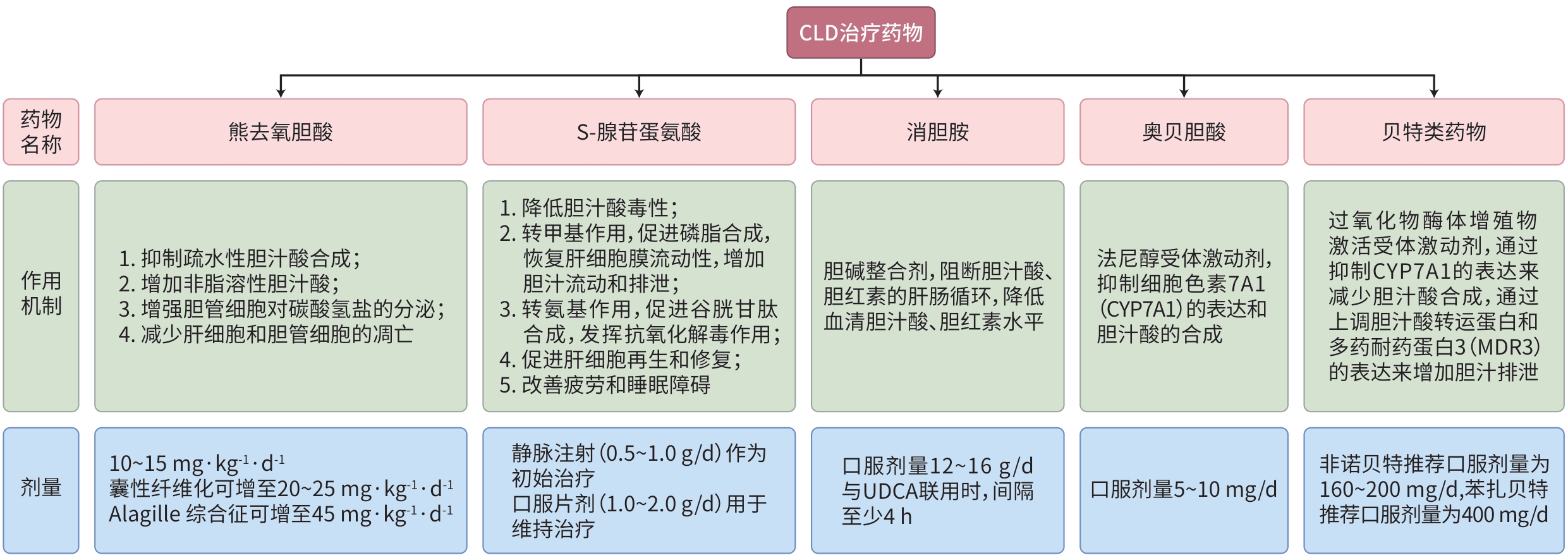
摘要: 胆汁淤积性肝病(CLD)是一类因胆汁生成、分泌或排泄障碍所致的肝胆疾病。随着液态活检、多参数影像学检查和基因组学技术的应用,CLD的诊断和预后评估取得了显著进展。目前,CLD的治疗原则包含去除病因和缓解胆汁淤积,常用药物包括熊去氧胆酸、S-腺苷蛋氨酸、消胆胺、贝特类药物和奥贝胆酸等。本文基于最新的临床共识和研究进展,系统阐述了CLD在诊断、治疗及预后评估方面的管理策略。Abstract: Cholestatic liver disease (CLD) is a group of hepatobiliary disorders caused by impaired bile production, secretion, or excretion. With the application of liquid biopsy, multi-parameter radiological examination, and genomics technology, significant progress has been made in the diagnosis and prognostic evaluation of CLD. At present, the therapeutic principles for CLD mainly focus on addressing the underlying causes and managing cholestasis, and commonly used drugs include ursodeoxycholic acid, S-adenosylmethionine, cholestyramine, fibrates, and obeticholic acid. Based on the latest clinical consensus statements and research advances in CLD, this article systematically elaborates on the clinical management strategies for CLD from the aspects of diagnosis, treatment, and prognostic evaluation.
-
Key words:
- Cholestasis /
- Ursodeoxycholic Acid /
- Obeticholic Acid
-
[1] LUO X, LU LG. Progress in the management of patients with cholestatic liver disease: Where are we and where are we going?[J]. J Clin Transl Hepatol, 2024, 12( 6): 581- 588. DOI: 10.14218/JCTH.2023.00519. [2] LU LG. Guidelines for the management of cholestatic liver diseases(2021)[J]. J Clin Transl Hepatol, 2022, 10( 4): 757- 769. DOI: 10.14218/jcth.2022.00147. [3] CHAZOUILLERES O, BEUERS U, BERGQUIST A, et al. EASL Clinical Practice Guidelines on sclerosing cholangitis[J]. J Hepatol, 2022, 77( 3): 761- 806. DOI: 10.1016/j.jhep.2022.05.011. [4] TANAKA A, MA X, TAKAHASHI A, et al. Primary biliary cholangitis[J]. Lancet, 2024, 404( 10457): 1053- 1066. DOI: 10.1016/S0140-6736(24)01303-5. [5] KARLSEN TH, FOLSERAAS T, THORBURN D, et al. Primary sclerosing cholangitis-a comprehensive review[J]. J Hepatol, 2017, 67( 6): 1298- 1323. DOI: 10.1016/j.jhep.2017.07.022. [6] BOWLUS CL, ARRIVÉ L, BERGQUIST A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma[J]. Hepatology, 2023, 77( 2): 659- 702. DOI: 10.1002/hep.32771. [7] MANNS MP, BERGQUIST A, KARLSEN TH, et al. Primary sclerosing cholangitis[J]. Nat Rev Dis Primers, 2025, 11: 17. DOI: 10.1038/s41572-025-00600-x. [8] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases[J]. J Hepatol, 2009, 51( 2): 237- 267. DOI: 10.1016/j.jhep.2009.04.009. [9] HARMS MH, van BUUREN HR, CORPECHOT C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2019, 71( 2): 357- 365. DOI: 10.1016/j.jhep.2019.04.001. [10] HIRSCHFIELD GM, DYSON JK, ALEXANDER GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines[J]. Gut, 2018, 67( 9): 1568- 1594. DOI: 10.1136/gutjnl-2017-315259. [11] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis[J]. J Hepatol, 2017, 67( 1): 145- 172. DOI: 10.1016/j.jhep.2017.03.022. [12] LINDOR KD, BOWLUS CL, BOYER J, et al. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases[J]. Hepatology, 2022, 75( 4): 1012- 1013. DOI: 10.1002/hep.32117. [13] HOSONUMA K, SATO K, YAMAZAKI Y, et al. A prospective randomized controlled study of long-term combination therapy using ursodeoxycholic acid and bezafibrate in patients with primary biliary cirrhosis and dyslipidemia[J]. Am J Gastroenterol, 2015, 110( 3): 423- 431. DOI: 10.1038/ajg.2015.20. [14] HONDA A, TANAKA A, KANEKO T, et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis[J]. Hepatology, 2019, 70( 6): 2035- 2046. DOI: 10.1002/hep.30552. [15] TANAKA A, HIROHARA J, NAKANO T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2021, 75( 3): 565- 571. DOI: 10.1016/j.jhep.2021.04.010. [16] van HOOFF MC, WERNER E, van der MEER AJ. Treatment in primary biliary cholangitis: Beyond ursodeoxycholic acid[J]. Eur J Intern Med, 2024, 124: 14- 21. DOI: 10.1016/j.ejim.2024.01.030. [17] SORET PA, LAM L, CARRAT F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis[J]. Aliment Pharmacol Ther, 2021, 53( 10): 1138- 1146. DOI: 10.1111/apt.16336. [18] ASSIS DN, BOWLUS CL. Recent advances in the management of primary sclerosing cholangitis[J]. Clin Gastroenterol Hepatol, 2023, 21( 8): 2065- 2075. DOI: 10.1016/j.cgh.2023.04.004. [19] OLSSON R, BOBERG KM, de MUCKADELL OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: A 5-year multicenter, randomized, controlled study[J]. Gastroenterology, 2005, 129( 5): 1464- 1472. DOI: 10.1053/j.gastro.2005.08.017. [20] IMAM MH, SINAKOS E, GOSSARD AA, et al. High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis[J]. Aliment Pharmacol Ther, 2011, 34( 10): 1185- 1192. DOI: 10.1111/j.1365-2036.2011.04863.x. [21] KOWDLEY KV, VUPPALANCHI R, LEVY C, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis[J]. J Hepatol, 2020, 73( 1): 94- 101. DOI: 10.1016/j.jhep.2020.02.033. [22] TABIBIAN JH, O’HARA SP, TRUSSONI CE, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis[J]. Hepatology, 2016, 63( 1): 185- 196. DOI: 10.1002/hep.27927. [23] DAVIES YK, COX KM, ABDULLAH BA, et al. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: An immunomodulating antibiotic[J]. J Pediatr Gastroenterol Nutr, 2008, 47( 1): 61- 67. DOI: 10.1097/MPG.0b013e31816fee95. [24] DENEAU MR, MACK C, MOGUL D, et al. Oral vancomycin, ursodeoxycholic acid, or No therapy for pediatric primary sclerosing cholangitis: A matched analysis[J]. Hepatology, 2021, 73( 3): 1061- 1073. DOI: 10.1002/hep.31560. [25] LAMMERS WJ, HIRSCHFIELD GM, CORPECHOT C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy[J]. Gastroenterology, 2015, 149( 7): 1804- 1812. e 4. DOI: 10.1053/j.gastro.2015.07.061. [26] GOET JC, MURILLO PEREZ CF, HARMS MH, et al. A comparison of prognostic scores(mayo, UK-PBC, and GLOBE) in primary biliary cholangitis[J]. Am J Gastroenterol, 2021, 116( 7): 1514- 1522. DOI: 10.14309/ajg.0000000000001285. [27] CARBONE M, SHARP SJ, FLACK S, et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis[J]. Hepatology, 2016, 63( 3): 930- 950. DOI: 10.1002/hep.28017. [28] HARMS MH, de VEER RC, LAMMERS WJ, et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis[J]. Gut, 2020, 69( 8): 1502- 1509. DOI: 10.1136/gutjnl-2019-319057. [29] MURILLO PEREZ CF, HARMS MH, LINDOR KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase[J]. Am J Gastroenterol, 2020, 115( 7): 1066- 1074. DOI: 10.14309/ajg.0000000000000557. [30] CORPECHOT C, LEMOINNE S, SORET PA, et al. Adequate versus deep response to ursodeoxycholic acid in primary biliary cholangitis: To what extent and under what conditions is normal alkaline phosphatase level associated with complication-free survival gain?[J]. Hepatology, 2024, 79( 1): 39- 48. DOI: 10.1097/HEP.0000000000000529. [31] CHEUNG AC, LAMMERS WJ, MURILLO PEREZ CF, et al. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis[J]. Clin Gastroenterol Hepatol, 2019, 17( 10): 2076- 2084. e 2. DOI: 10.1016/j.cgh.2018.12.028. [32] CORPECHOT C, CARRAT F, GAOUAR F, et al. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis[J]. J Hepatol, 2022, 77( 6): 1545- 1553. DOI: 10.1016/j.jhep.2022.06.017. [33] CRISTOFERI L, CALVARUSO V, OVERI D, et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in Naïve patients with primary biliary cholangitis: A dual cut-off approach[J]. Hepatology, 2021, 74( 3): 1496- 1508. DOI: 10.1002/hep.31810. [34] KIM WR, THERNEAU TM, WIESNER RH, et al. A revised natural history model for primary sclerosing cholangitis[J]. Mayo Clin Proc, 2000, 75( 7): 688- 694. DOI: 10.4065/75.7.688. [35] GOODE EC, CLARK AB, MELLS GF, et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system[J]. Hepatology, 2019, 69( 5): 2120- 2135. DOI: 10.1002/hep.30479. [36] de VRIES EM, WANG JF, WILLIAMSON KD, et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis[J]. Gut, 2018, 67( 10): 1864- 1869. DOI: 10.1136/gutjnl-2016-313681. [37] EATON JE, VESTERHUS M, MCCAULEY BM, et al. Primary sclerosing cholangitis risk estimate tool(PREsTo) predicts outcomes of the disease: A derivation and validation study using machine learning[J]. Hepatology, 2020, 71( 1): 214- 224. DOI: 10.1002/hep.30085. [38] DENEAU MR, MACK C, PERITO ER, et al. The sclerosing cholangitis outcomes in pediatrics(SCOPE) index: A prognostic tool for children[J]. Hepatology, 2021, 73( 3): 1074- 1087. DOI: 10.1002/hep.31393. -



 PDF下载 ( 1018 KB)
PDF下载 ( 1018 KB)


 下载:
下载: