107例原发性硬化性胆管炎患者的临床特征与预后分析
DOI: 10.12449/JCH250717
Clinical features and prognosis of patients with primary sclerosing cholangitis: An analysis of 107 cases
-
摘要:
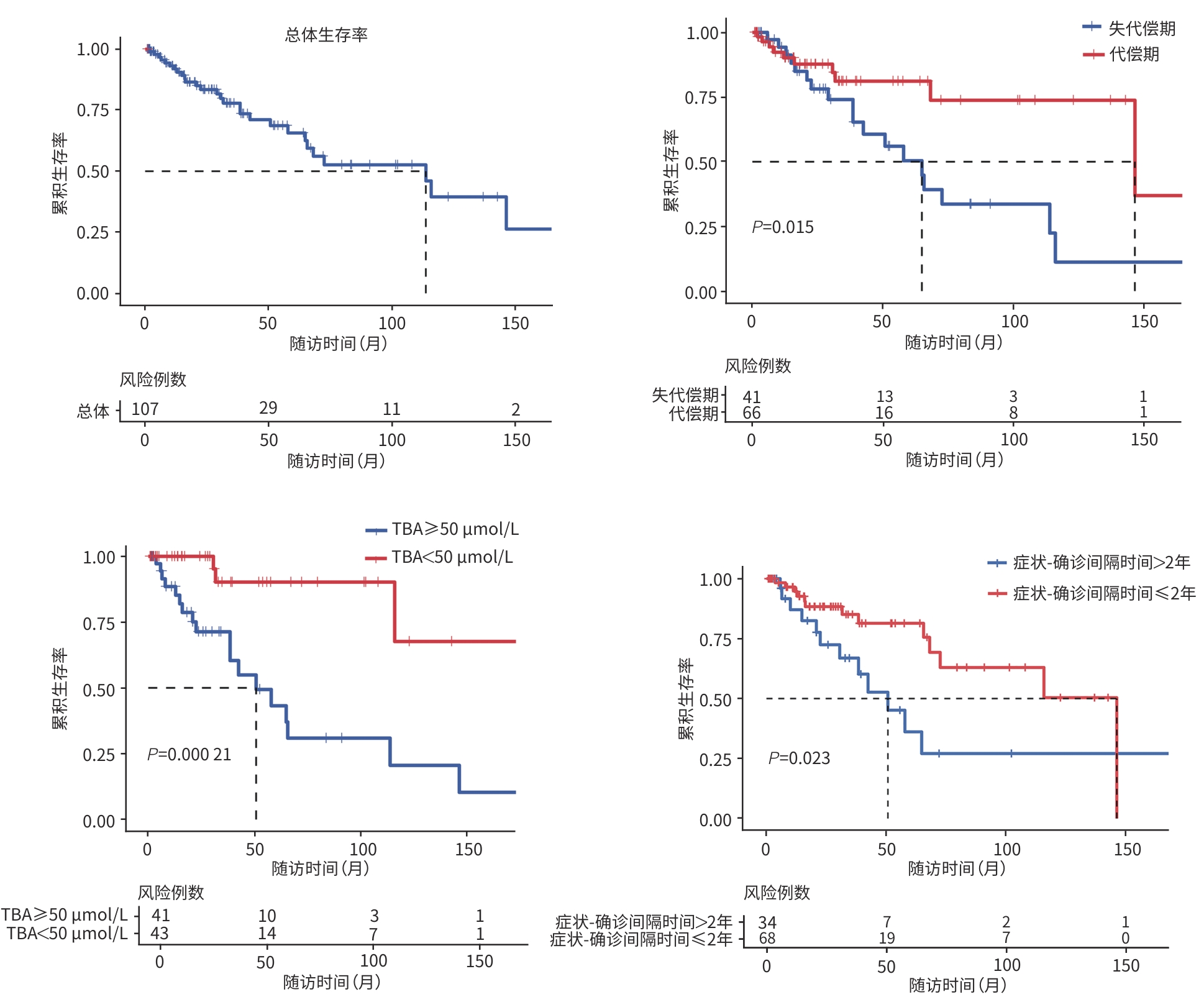
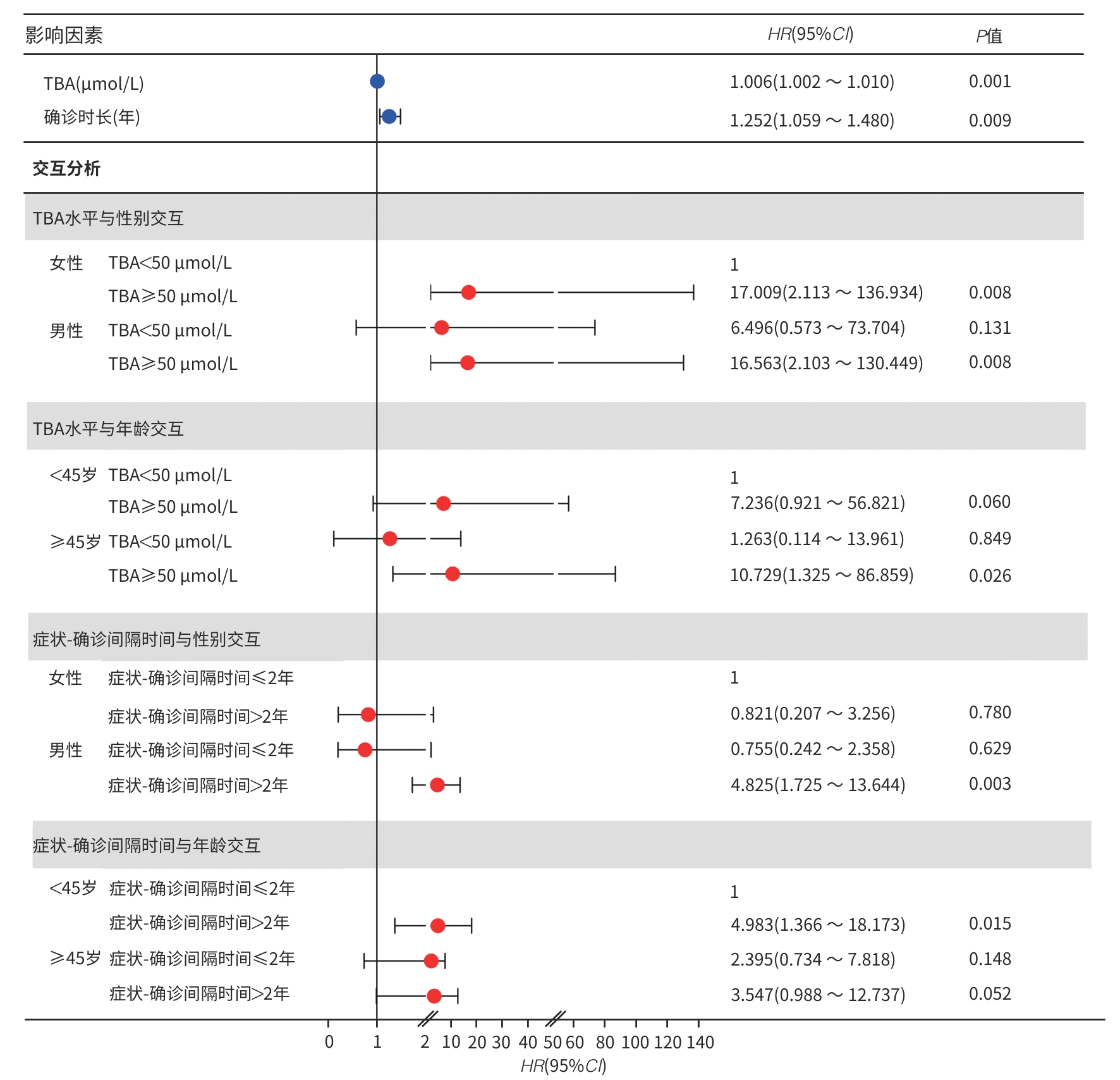
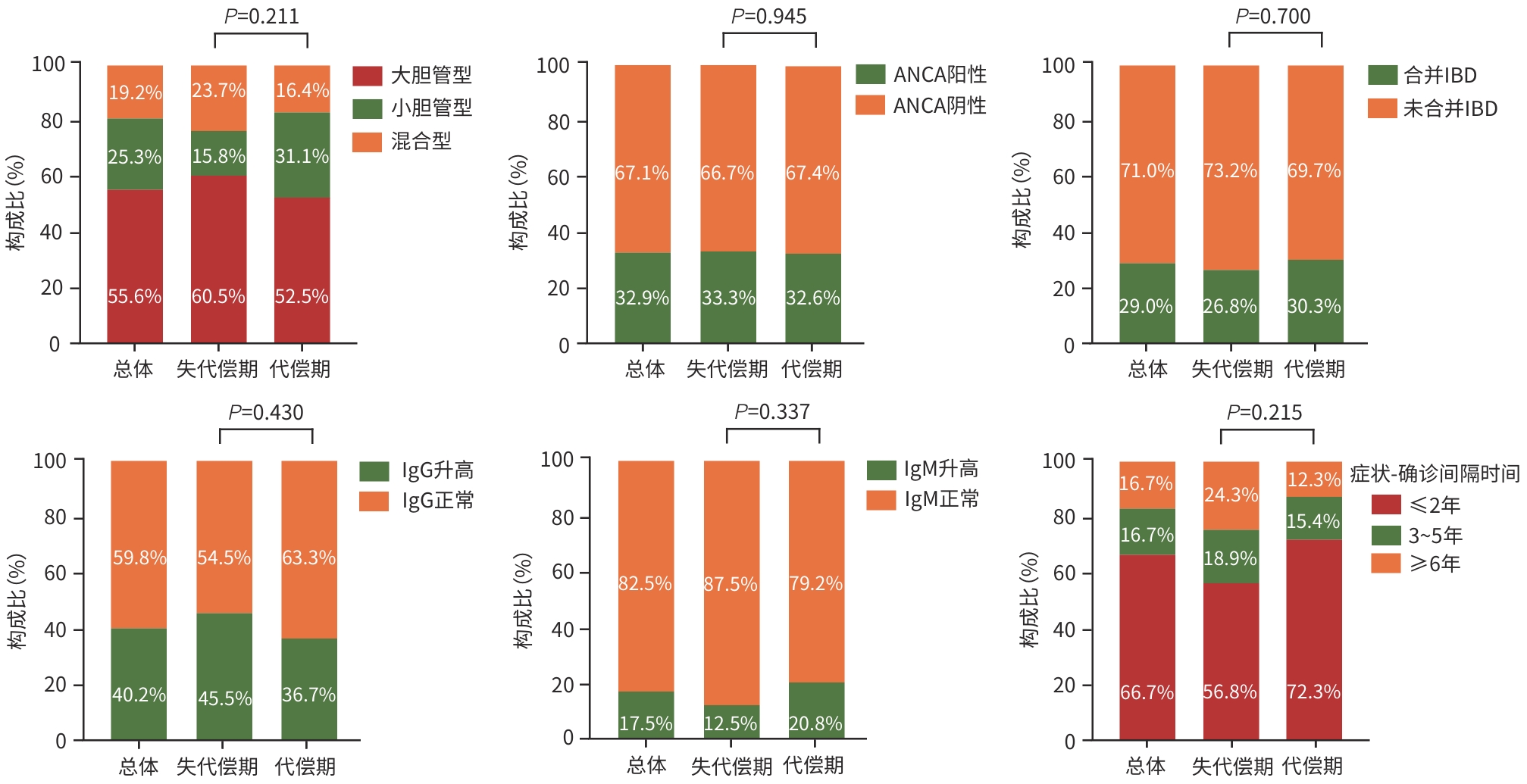
目的 基于全国多中心患者队列,描述我国原发性硬化性胆管炎(PSC)患者的临床特征,并探讨影响其预后的相关危险因素。 方法 采用回顾性队列研究设计,基于全国7家三级甲等医疗中心的电子病历系统检索出明确诊断为PSC的患者,并提取研究数据。计量资料组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。采用Kaplan-Meier法估算患者无肝移植生存率,Log-rank检验比较不同特征PSC患者生存率的统计学差异。采用Cox回归模型识别影响PSC患者预后的独立危险因素及关键因素的交互作用。 结果 共纳入107例PSC患者,其中55.6%(55/99)为大胆管型,29.0%(31/107)合并炎症性肠病(IBD),血清抗中性粒细胞胞浆抗体(ANCA)阳性率为32.9%(24/73),50.0%(40/80)伴有免疫球蛋白(Ig)G/IgM升高,症状出现至确诊的中位时间(症状-确诊间隔时间)为1年(<1~4.0),38.3%(41/107)的患者在确诊时已进展至失代偿期肝硬化。患者总体中位无肝移植生存期为114(95%CI:62~166)个月,5年累积生存率为65.7%。多因素分析结果显示,总胆汁酸(TBA)水平升高(HR=1.006,95%CI:1.002~1.010,P=0.001)和症状-确诊间隔时间延长(HR=1.252,95%CI:1.059~1.480,P=0.009)是影响预后的独立危险因素。交互分析结果显示,与TBA<50 μmol/L的女性患者相比,TBA≥50 μmol/L的男性患者(HR=16.563,95%CI:2.103~130.449)和女性患者(HR=17.009,95%CI:2.113~136.934)的肝移植或死亡风险均显著增加(P值均<0.001);与<45岁且TBA<50 μmol/L的患者相比,年龄≥45岁且TBA≥50 μmol/L的患者(HR=10.729,95%CI:1.325~86.859)的肝移植或死亡风险显著增加(P=0.026)。与症状-确诊间隔时间≤2年的女性患者相比,症状-确诊间隔时间>2年的男性患者(HR=4.825,95%CI:1.725~13.644)的肝移植或死亡风险增加(P=0.003);与<45岁且症状-确诊间隔时间≤2年的患者相比,<45岁且症状-确诊间隔时间>2年的患者(HR=4.983,95%CI:1.366~18.173)的肝移植或死亡风险增加(P=0.015)。 结论 与西方报道相比,我国PSC亦以大胆管型为主但占比相对较低,合并IBD者和ANCA阳性比例亦低。TBA水平升高和症状-确诊间隔延长是独立预后危险因素,且与年龄、性别存在显著交互作用。提示需加强早期筛查和干预以改善预后。 Abstract:Objective To describe the clinical features of patients with primary sclerosing cholangitis (PSC) in China based on a nationwide multicenter patient cohort, and to investigate the risk factors for prognosis. Methods A retrospective cohort study was conducted among the patients with a confirmed diagnosis of PSC based on the electronic medical record system of seven grade A tertiary hospitals across the country, and related data were extracted. The Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. The Kaplan-Meier method was used to estimate liver transplant-free survival, and the log-rank test was used for comparison of survival rate between PSC patients with different features. The Cox regression model was used to identify independent risk factors for the prognosis of PSC patients and the interactions between key factors. Results A total of 107 patients were enrolled, among whom 55.6% (55/99) had large-duct PSC and 29.0% (31/107) had comorbidity with inflammatory bowel disease (IBD). The positivity rate of anti-neutrophil cytoplasmic antibody (ANCA) was 32.9% (24/73), and 50.0% (40/80) of the patients had an increase in IgG/IgM. The median symptom-to-diagnosis interval was 1 year (<1 — 4.0), and 38.3% (41/107) of the patients had progressed to decompensated cirrhosis at the time of diagnosis. The median liver transplant-free survival time was 114 months (95% confidence interval [CI]:62 — 166), with a 5-year survival rate of 65.7%. The multivariate analysis showed that an increase in total bile acid (TBA) (hazard ratio [HR]=1.006,95%CI:1.002 — 1.010,P=0.001) and a prolonged symptom-to-diagnosis interval (HR=1.252,95%CI:1.059 — 1.480,P=0.009) were independent risk factors for prognosis. The interaction analysis showed that compared with the female patients with TBA<50 μmol/L, both male and female patients with TBA≥50 μmol/L had a significant increase in the risk of liver transplantation or death (male:HR=16.563,95%CI:2.103 — 130.449,P<0.001; female: HR=17.009,95%CI:2.113 — 136.934,P<0.001), and compared with the patients with an age of <45 years and a TBA level of <50 μmol/L, the patients with an age of ≥45 years and a TBA level of≥50 μmol/L had a significant increase in the risk of liver transplantation or death (HR=10.729,95%CI:1.325 — 86.859,P=0.026). Compared with the female patients with an symptom-to-diagnosis interval of≤2 years, the male patients with a symptom-to-diagnosis interval of>2 years had an increased risk of liver transplantation or death (HR=4.825,95%CI:1.725 — 13.644,P=0.003), and compared with the patients with an age of<45 years and a symptom-to-diagnosis interval of≤2 years, the patients with an age of<45 years and a symptom-to-diagnosis interval of>2 years had an increased risk of liver transplantation or death (HR=4.983,95%CI:1.366 — 18.173,P=0.015). Conclusion Compared with the reports from Western countries, large-duct PSC is also the main type of PSC in China, but with a relatively low proportion, and there is also a relatively low proportion of patients with IBD or positive ANCA. An increase in TBA and a prolonged symptom-to-diagnosis interval are independent risk factors for prognosis, with significant interactions with age and sex. This suggests that early screening and intervention should be enhanced to improve prognosis. -
Key words:
- Cholangitis, Sclerosing /
- Clinical Features /
- Prognosis
-
表 1 107例PSC患者的基本特征
Table 1. The demographic and clinical characteristics of 107 patients with PSC
特征 总体(n=107) 失代偿期(n=41) 代偿期(n=66) 统计值 P值 人口学资料 男[例(%)] 52(48.6) 21(51.2) 31(47.0) χ2=0.183 0.669 诊断时年龄(岁) 47(36~56) 47(34~57) 47(36~57) Z=-0.157 0.875 临床指标 ALT(U/L) 74.5(37.3~127.8) 68.0(42.0~104.0) 79.0(36.1~146.1) Z=-1.145 0.252 AST(U/L) 77.0(44.2~117.8) 77.4(52.0~114.9) 71.3(36.1~125.3) Z=-0.339 0.735 ALP(U/L) 285.0(206.8~432.0) 285.0(208.0~412.0) 284.5(202.5~511.9) Z=-0.241 0.809 GGT(U/L) 217.4(117.0~377.1) 185.0(93.0~259.0) 305.0(124.5~508.5) Z=-2.092 0.036 Alb(g/L) 36.4(32.0~39.9) 33.5(30.4~37.7) 38.2(33.0~41.0) Z=-3.166 0.002 TBil(μmol/L) 33.4(15.0~99.7) 55.7(23.2~125.7) 23.9(13.2~93.1) Z=-1.846 0.065 TBA(μmol/L) 44.8(15.1~131.5) 93.3(42.7~140.2) 27.0(12.5~123.0) Z=-2.666 0.008 症状-确诊间隔时间(年) 1.0(<1~4.0) 2.0(<1~5.5) 1.0(<1~3) Z=-1.455 0.146 -
[1] MEHTA TI, WEISSMAN S, FUNG BM, et al. Global incidence, prevalence and features of primary sclerosing cholangitis: A systematic review and meta-analysis[J]. Liver Int, 2021, 41( 10): 2418- 2426. DOI: 10.1111/liv.15007. [2] ANG TL, FOCK KM, NG TM, et al. Clinical profile of primary sclerosing cholangitis in Singapore[J]. J Gastroenterol Hepatol, 2002, 17( 8): 908- 913. DOI: 10.1046/j.1440-1746.2002.02835.x. [3] XU XQ, MENG TT, SHI LC, et al. Prevalence and clinical profiles of primary sclerosing cholangitis in China: Data from electronic medical records and systematic literature retrieval[J]. J Autoimmun, 2024, 147: 103264. DOI: 10.1016/j.jaut.2024.103264. [4] SHI X, WANG XP, ZHANG Y, et al. Endoscopic treatment and prognosis of primary sclerosing cholangitis[J]. Chin J Dig Endosc, 2022, 39( 12): 992- 997. DOI: 10.3760/cma.j.cn321463-20210709-00349.史鑫, 王向平, 张妍, 等. 原发性硬化性胆管炎的内镜治疗及预后分析[J]. 中华消化内镜杂志, 2022, 39( 12): 992- 997. DOI: 10.3760/cma.j.cn321463-20210709-00349. [5] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [6] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management of primary sclerosing cholangitis(2021)[J]. J Clin Hepatol, 2022, 38( 1): 50- 61. DOI: 10.3760/cma.j.cn112138-20211109-00786.中华医学会肝病学分会. 原发性硬化性胆管炎诊断及治疗指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 50- 61. DOI: 10.3760/cma.j.cn112138-20211109-00786. [7] WEISMÜLLER TJ, TRIVEDI PJ, BERGQUIST A, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis[J]. Gastroenterology, 2017, 152( 8): 1975- 1984. e 8. DOI: 10.1053/j.gastro.2017.02.038. [8] CHEN S, MENG TT, DUAN WJ, et al. Characteristics and prognosis of patients with primary sclerosing cholangitis[J]. Chin J Intern Med, 2025, 64( 3): 206- 211. DOI: 10.3760/cma.j.cn112138-20241008-00663.陈莎, 孟彤彤, 段维佳, 等. 原发性硬化性胆管炎患者的疾病特点及预后分析[J]. 中华内科杂志, 2025, 64( 3): 206- 211. DOI: 10.3760/cma.j.cn112138-20241008-00663. [9] TANAKA A, TAZUMA S, OKAZAKI K, et al. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan[J]. J Hepatobiliary Pancreat Sci, 2014, 21( 1): 43- 50. DOI: 10.1002/jhbp.50. [10] TAKIKAWA H. Characteristics of primary sclerosing cholangitis in Japan[J]. Hepatol Res, 2007, 37( s3): S470- S473. DOI: 10.1111/j.1872-034X.2007.00241.x. [11] TOY E, BALASUBRAMANIAN S, SELMI C, et al. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population[J]. BMC Gastroenterol, 2011, 11: 83. DOI: 10.1186/1471-230X-11-83. [12] TRIVEDI PJ, BOWLUS CL, YIMAM KK, et al. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: A systematic review of population-based studies[J]. Clin Gastroenterol Hepatol, 2022, 20( 8): 1687- 1700. e 4. DOI: 10.1016/j.cgh.2021.08.039. [13] LIAN M, LI B, XIAO X, et al. Comparative clinical characteristics and natural history of three variants of sclerosing cholangitis: IgG4-related SC, PSC/AIH and PSC alone[J]. Autoimmun Rev, 2017, 16( 8): 875- 882. DOI: 10.1016/j.autrev.2017.05.018. [14] TERJUNG B, SPENGLER U. Role of auto-antibodies for the diagnosis of chronic cholestatic liver diseases[J]. Clin Rev Allergy Immunol, 2005, 28( 2): 115- 133. DOI: 10.1385/CRIAI:28:2:115. [15] KARLSEN TH, FOLSERAAS T, THORBURN D, et al. Primary sclerosing cholangitis-a comprehensive review[J]. J Hepatol, 2017, 67( 6): 1298- 1323. DOI: 10.1016/j.jhep.2017.07.022. [16] European Association for the Study of the Liver. EASL clinical practice guidelines on sclerosing cholangitis[J]. J Hepatol, 2022, 77( 3): 761- 806. DOI: 10.1016/j.jhep.2022.05.011. [17] TANAKA A, TAZUMA S, NAKAZAWA T, et al. No negative impact of serum IgG4 levels on clinical outcome in 435 patients with primary sclerosing cholangitis from Japan[J]. J Hepatobiliary Pancreat Sci, 2017, 24( 4): 217- 225. DOI: 10.1002/jhbp.432. -



 PDF下载 ( 1664 KB)
PDF下载 ( 1664 KB)

 下载:
下载: