甲磺酸阿帕替尼联合吉西他滨+顺铂+卡瑞利珠单抗治疗晚期胆囊癌的效果及安全性分析
DOI: 10.12449/JCH250726
Effectiveness and safety of apatinib mesylate combined with gemcitabine+cisplatin+camrelizumab in patients with advanced gallbladder cancer
-
摘要:
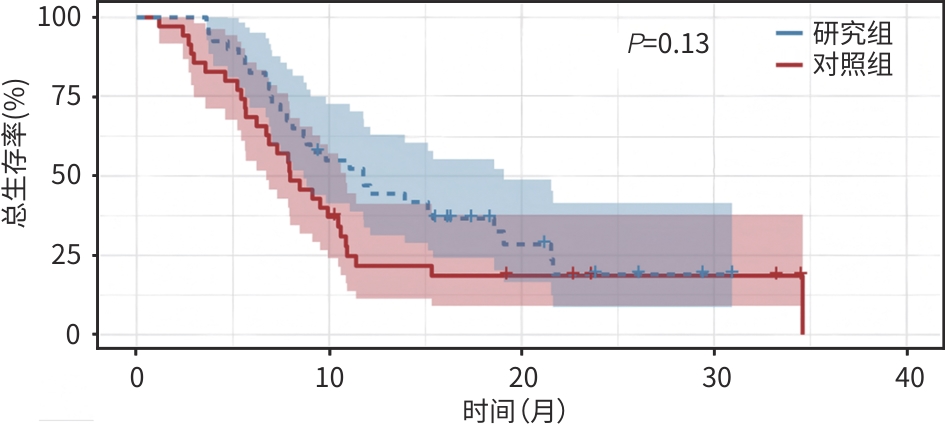
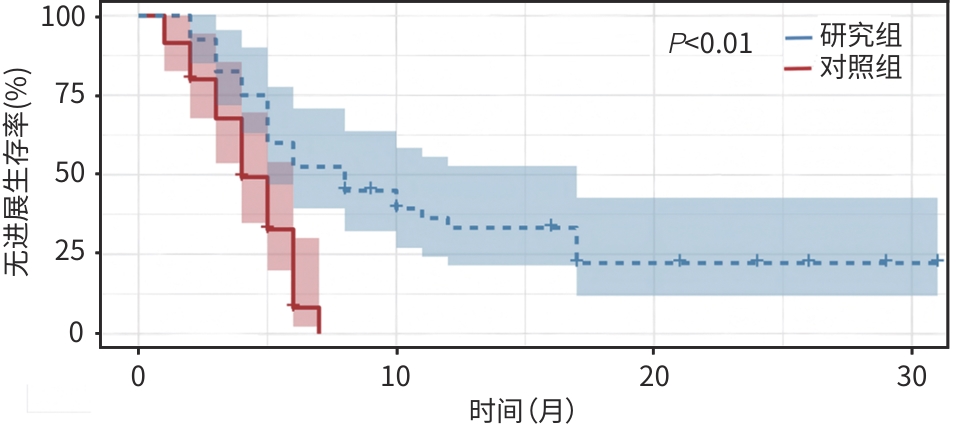
目的 探究甲磺酸阿帕替尼联合吉西他滨+顺铂(GC)及卡瑞利珠单抗治疗晚期胆囊癌的疗效及安全性,为晚期胆囊癌患者的临床用药提供相关依据。 方法 回顾性纳入了2022年1月—2023年12月就诊于郑州大学人民医院及郑州大学附属肿瘤医院的75例晚期胆囊癌患者,根据治疗方法分为研究组(甲磺酸阿帕替尼联合GC+卡瑞利珠单抗)及对照组(GC+卡瑞利珠单抗),比较两组患者1年生存率、客观缓解率(ORR)、疾病控制率(DCR)、无进展生存期(PFS)、总生存期(OS)和不良反应发生情况。计数资料两组间比较采用χ2检验或Fisher确切检验,符合正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验。生存分析采用Kaplan-Meier法,生存曲线差异比较采用Log-rank检验。 结果 研究组ORR和DCR分别为35.0%、80.0%,与对照组比较差异均无统计学意义(P值均>0.05)。研究组1年生存率达45.0%,与对照组(20.0%)比较差异有统计学意义(χ²=5.25,P<0.05)。研究组中位PFS为7.73个月(95%CI:4.39~11.01),对照组为4.17个月(95%CI:3.48~4.85),差异有统计学意义(χ²=16.735,P<0.01)。研究组中位OS为11.77个月(95%CI:8.07~15.47),对照组为7.97个月(95%CI:5.84~10.09),差异无统计学意义(P>0.05)。任何级别不良反应中,研究组患者手足综合征(62.5%)、血压升高(42.5%)的发生率均明显高于对照组(34.3%、20.0%),差异均具有统计学意义(χ²值分别为5.945、4.343,P值均<0.05);≥Ⅲ级不良反应的发生率在两组间差异无统计学意义(P值均>0.05)。 结论 甲磺酸阿帕替尼联合GC+卡瑞利珠单抗治疗方案在延长晚期胆囊癌患者PFS方面较GC+卡瑞利珠单抗治疗方案更具优势,但对OS的影响二者无明显差异,其毒副反应可控,是一种安全、有效的治疗方案。 Abstract:Objective To investigate the clinical effectiveness and safety of apatinib mesylate combined with gemcitabine+cisplatin (GC) and camrelizumab in the treatment of advanced gallbladder cancer, and to provide evidence for the clinical treatment of patients with advanced gallbladder cancer. Methods A total of 75 patients with advanced gallbladder cancer admitted to Henan Provincial People’s Hospital and The Affiliated Cancer Hospital of Zhengzhou University from January 2022 to December 2023 were retrospectively included. According to treatment plans, they were divided into study group (apatinib mesylate combined with GC+camrelizumab) and control group (GC+camrelizumab). The 1-year survival rate, objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse reactions were compared between the two groups. Inter-group comparisons were performed using the chi-square test/Fisher’s exact test, the t-test, and the Mann-Whitney U test for categorical data, continuous data in normal distribution, and continuous data in non-normal distribution, respectively. The Kaplan-Meier survival curves were generated and compared using the log-rank test. Results The ORR and DCR of the study group were 35.0% and 80.0%, respectively, which were not significantly different from those of the control group (bothP>0.05). The 1-year survival rate of the study group differed significantly from that of the control group (45.0% vs 20.0%,P<0.05). The median PFS was 7.73 (95% confidence interval [CI]:4.39 — 11.01) months in the study group and 4.17 (95%CI:3.48 — 4.85) months in the control group, and the difference was statistically significant (P<0.01). The median OS was 11.77 (95%CI:8.07 — 15.47) months in the study group and 7.97 (95%CI:5.84 — 10.09) months in the control group, which were not statistically significant (P>0.05). Across all grades of adverse reactions, the study group showed significantly higher incidence rates of hand-foot syndrome (62.5% vs 34.3%,χ2=5.945,P<0.05) and elevated blood pressure (42.5% vs 20.0%,χ2=4.343,P<0.05) than the control group. There were no significant differences in the incidence rates of adverse reactions of grade Ⅲ or above between the two groups (all P>0.05). Conclusion Apatinib mesylate combined with GC+camrelizumab is superior to GC+camrelizumab in prolonging the PFS but not in terms of the OS, with controllable toxic side effects, which is a safe and effective treatment regimen. -
表 1 两组患者基线资料比较
Table 1. Comparison of baseline characteristics between the two groups
组别 研究组
(n=40)对照组
(n=35)P值 年龄[例(%)] 0.772 <60岁 15(37.5) 12(34.3) ≥60岁 25(62.5) 23(65.7) 性别[例(%)] 0.308 男 23(57.5) 16(45.7) 女 17(42.5) 19(54.3) BMI[例(%)] 0.975 <24 kg/m² 23(57.5) 20(57.1) ≥24 kg/m² 17(42.5) 15(42.9) ECOG[例(%)] 0.967 0~1分 33(82.5) 29(82.9) 2分 7(17.5) 6(17.1) 转移部位[例(%)] 0.971 肝脏和淋巴结转移 31(77.5) 27(77.1) 远处转移 9(22.5) 8(22.9) 行姑息性手术切除[例(%)] 0.486 是 26(65.0) 20(57.1) 否 14(35.0) 15(42.9) 表 2 两组患者mRESIST疗效评价
Table 2. Efficacy evaluation of mrecist between the two groups
组别 研究组(n=40) 对照组(n=35) χ2值 P值 CR[例(%)] 1(2.5) 0(0.0) PR[例(%)] 13(32.5) 6(17.1) SD[例(%)] 18(45.0) 17(48.6) PD[例(%)] 8(20.0) 12(34.3) ORR[例(%)] 14(35.0) 6(17.1) 3.044 0.081 DCR[例(%)] 32(80.0) 23(65.7) 1.948 0.163 表 3 两组患者不良反应比较
Table 3. Comparison of adverse reactions between the two groups
项目 任何级别不良反应 ≥Ⅲ级不良反应 研究组(n=40) 对照组(n=35) χ2值 P值 研究组(n=40) 对照组(n=35) χ2值 P值 恶心呕吐[例(%)] 30(75.0) 28(80.0) 0.266 0.606 2(5.0) 2(5.7) 0.019 0.891 便秘腹泻[例(%)] 26(65.0) 29(82.9) 3.044 0.081 1(2.5) 2(5.7) 0.502 0.479 手足综合征[例(%)] 25(62.5) 12(34.3) 5.945 <0.05 6(15.0) 2(5.7) 1.689 0.194 蛋白尿[例(%)] 7(17.5) 3(8.6) 1.288 0.256 0(0.0) 0(0.0) 骨髓抑制[例(%)] 33(82.5) 28(80.0) 0.077 0.782 5(12.5) 4(11.4) 0.020 0.887 血压升高[例(%)] 17(42.5) 7(20.0) 4.343 <0.05 2(5.0) 1(2.9) 0.223 0.637 -
[1] ROA JC, GARCÍA P, KAPOOR VK, et al. Gallbladder cancer[J]. Nat Rev Dis Primers, 2022, 8( 1): 69. DOI: 10.1038/s41572-022-00398-y. [2] YIN XY, XU QC. Surgical treatment of gallbladder cancer: Current status and advances[J]. J Clin Hepatol, 2024, 40( 12): 2366- 2370. DOI: 10.12449/JCH241205.殷晓煜, 许琼聪. 胆囊癌手术治疗的现状与进展[J]. 临床肝胆病杂志, 2024, 40( 12): 2366- 2370. DOI: 10.12449/JCH241205. [3] VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/NEJMoa0908721. [4] DU LW, LIU JS. Research advances in targeted therapy for gallbladder carcinoma[J]. J Clin Hepatol, 2023, 39( 12): 2965- 2969. DOI: 10.3969/j.issn.1001-5256.2023.12.032.杜丽文, 刘建生. 胆囊癌的靶向治疗研究进展[J]. 临床肝胆病杂志, 2023, 39( 12): 2965- 2969. DOI: 10.3969/j.issn.1001-5256.2023.12.032. [5] YI M, ZHENG X, NIU M, et al. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions[J]. Mol Cancer, 2022, 21( 1): 28. DOI: 10.1186/s12943-021-01489-2. [6] OH DY, HE AR, QIN SK, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin(GemCis) in patients(pts) with advanced biliary tract cancer(BTC): TOPAZ-1[J]. J Clin Oncol, 2022, 40( 4_suppl): 378. DOI: 10.1200/jco.2022.40.4_suppl.378. [7] KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/S0140-6736(23)00727-4. [8] LIU YB, CHEN W. Attach importance to the clinical and basic research on gallbladder carcinoma[J]. Chin J Dig Surg, 2025, 24( 1): 64- 71. DOI: 10.3760/cma.j.cn115610-20241227-00588.刘颖斌, 陈炜. 重视胆囊癌的临床和基础研究[J]. 中华消化外科杂志, 2025, 24( 1): 64- 71. DOI: 10.3760/cma.j.cn115610-20241227-00588. [9] LIU ZL, CHEN HH, ZHENG LL, et al. Angiogenic signaling pathways and anti-angiogenic therapy for cancer[J]. Signal Transduct Target Ther, 2023, 8( 1): 198. DOI: 10.1038/s41392-023-01460-1. [10] LIANG JM, GU WG, JIN J, et al. Efficacy and safety of apatinib as third-or further-line therapy for patients with advanced NSCLC: A retrospective study[J]. Ther Adv Med Oncol, 2020, 12: 1758835920968472. DOI: 10.1177/1758835920968472. [11] YUAN S, FU Q, ZHAO L, et al. Efficacy and safety of apatinib in patients with recurrent or refractory melanoma[J]. Oncologist, 2022, 27( 6): e463- e470. DOI: 10.1093/oncolo/oyab068. [12] HU JK, LI XM, WANG YP, et al. SOX combined with apatinib and camrelizumab in the treatment of resectable locally advanced gastric cancer: A case report[J]. Front Immunol, 2024, 15: 1410284. DOI: 10.3389/fimmu.2024.1410284. [13] YANG YJ, KE TY, LIU SX, et al. Synergistic sensitization of apatinib mesylate and radiotherapy on hepatocarcinoma cells in vitro[J]. J Jilin Univ Med Ed, 2024, 50( 4): 1009- 1015. DOI: 10.13481/j.1671-587X.202404015.杨永净, 柯天洋, 刘士新, 等. 甲磺酸阿帕替尼联合放疗对肝癌HepG2细胞的体外协同增敏作用[J]. 吉林大学学报(医学版), 2024, 50( 4): 1009- 1015. DOI: 10.13481/j.1671-587X.202404015. [14] WANG DX, YANG X, LONG JY, et al. The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: A prospective clinical study[J]. Front Oncol, 2021, 11: 646979. DOI: 10.3389/fonc.2021.646979. [15] RAO JH, WU C, ZHANG H, et al. Efficacy and biomarker analysis of neoadjuvant carrizumab plus apatinib in patients with local advanced biliary tract cancers[J]. J Clin Oncol, 2021, 39( 15_suppl): e16126. DOI: 10.1200/jco.2021.39.15_suppl.e16126. [16] EISENHAUER EA, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline(version 1.1)[J]. Eur J Cancer, 2009, 45( 2): 228- 247. DOI: 10.1016/j.ejca.2008.10.026. [17] SUN XH, REN T, GENG YJ, et al. A largescale multicenter retrospective study of current surgical treatment modalities and pathological characteristics of patients with gallbladder cancer in China: A report of 4, 345 cases[J]. Chin J Pract Surg, 2021, 41( 1): 99- 106. DOI: 10.19538/j.cjps.issn1005-2208.2021.01.18.孙旭恒, 任泰, 耿亚军, 等. 中国胆囊癌外科治疗现状与病理学特征多中心回顾性研究(附4 345例报告)[J]. 中国实用外科杂志, 2021, 41( 1): 99- 106. DOI: 10.19538/j.cjps.issn1005-2208.2021.01.18. [18] RIZZO A, RICCI AD, BRANDI G. Recent advances of immunotherapy for biliary tract cancer[J]. Expert Rev Gastroenterol Hepatol, 2021, 15( 5): 527- 536. DOI: 10.1080/17474124.2021.1853527. [19] ZHAO HT, WANG SS. Critical issues in conversion therapy and sequential surgical management of gallbladder carcinoma[J]. Chin J Pract Surg, 2023, 43( 11): 1214- 1222. DOI: 10.19538/j.cjps.issn1005-2208.2023.11.03.赵海涛, 王闪闪. 胆囊癌转化治疗及序贯手术治疗的几个关键问题[J]. 中国实用外科杂志, 2023, 43( 11): 1214- 1222. DOI: 10.19538/j.cjps.issn1005-2208.2023.11.03. [20] LI H. 65P A single-arm, open-label, phase II study of tislelizumab combined with lenvatinib and Gemox regimen for conversion therapy of potentially resectable locally advanced biliary tract cancers[J]. Ann Oncol, 2022, 33: S570. DOI: 10.1016/j.annonc.2022.07.093. [21] SHI GM, HUANG XY, WU D, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study[J]. Signal Transduct Target Ther, 2023, 8( 1): 106. DOI: 10.1038/s41392-023-01317-7. [22] ZHU CP, XUE JN, WANG YC, et al. Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer[J]. Front Immunol, 2023, 14: 1109292. DOI: 10.3389/fimmu.2023.1109292. [23] GUPTA P, CANONICO ME, FAABORG-ANDERSEN C, et al. Updates in the management of cancer therapy-related hypertension[J]. Curr Opin Cardiol, 2024, 39( 4): 235- 243. DOI: 10.1097/HCO.0000000000001127. [24] MCLELLAN B, CIARDIELLO F, LACOUTURE ME, et al. Regorafenib-associated hand-foot skin reaction: Practical advice on diagnosis, prevention, and management[J]. Ann Oncol, 2015, 26( 10): 2017- 2026. DOI: 10.1093/annonc/mdv244. [25] VERSMISSEN J, MIRABITO COLAFELLA KM, KOOLEN SLW, et al. Vascular cardio-oncology: Vascular endothelial growth factor inhibitors and hypertension[J]. Cardiovasc Res, 2019, 115( 5): 904- 914. DOI: 10.1093/cvr/cvz022. [26] XIA H, ZHOU C, LUO ZX, et al. Apatinib-induced hand-foot skin reaction in Chinese patients with liver cancer[J]. Front Oncol, 2021, 11: 624369. DOI: 10.3389/fonc.2021.624369. -



 PDF下载 ( 2181 KB)
PDF下载 ( 2181 KB)

 下载:
下载: