聚乙二醇干扰素α-2b治疗HBeAg阴性慢性乙型肝炎患者发生HBsAg清除的影响因素以及预测模型的构建
DOI: 10.12449/JCH250810
Analysis of influencing factors and construction of predictive model for HBsAg clearance in patients with HBeAg-negative chronic hepatitis B treated with PEG-IFN-α-2b
-
摘要:
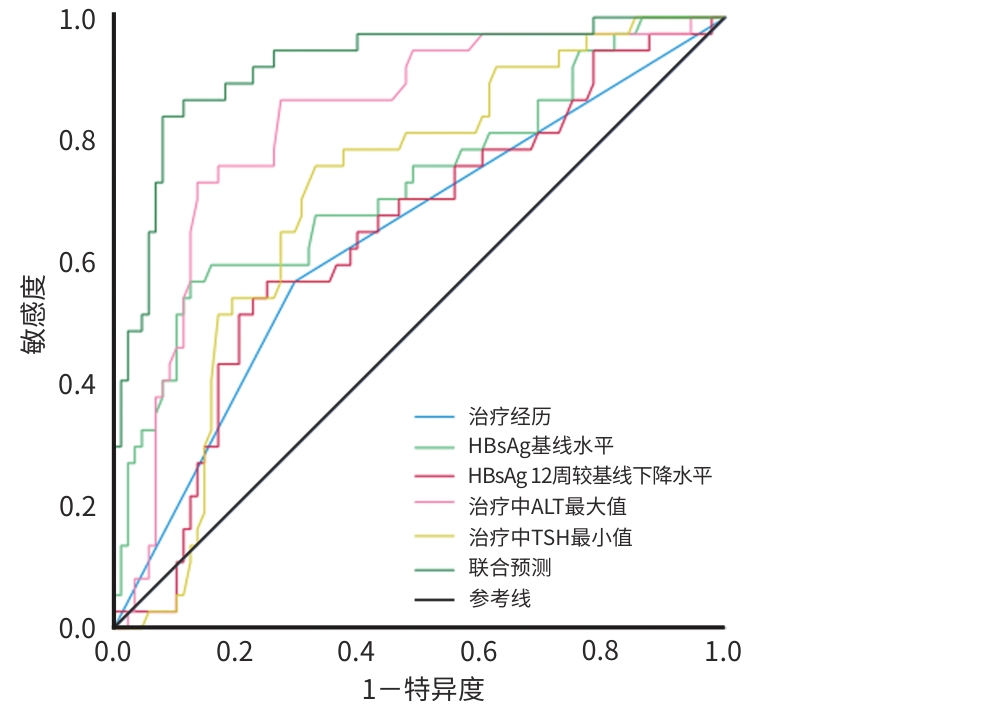
目的 探讨HBeAg阴性慢性乙型肝炎(CHB)患者在接受聚乙二醇干扰素(PEG-IFN-α-2b)治疗后发生HBsAg清除的预测因素,比较不同特征下各指标对HBsAg清除率的影响,建立联合预测模型,并评估模型的诊断价值。 方法 收集2021年5月—2023年5月于昆明市第三人民医院就诊的HBeAg阴性的CHB患者,共125例,入组患者使用PEG-IFN-α-2b联合核苷(酸)类似物(NUC)治疗,疗程满48周,分为HBsAg清除组和HBsAg未清除组,分别观察患者一般资料和治疗中不同时间节点的血清学生化指标、病毒学指标。符合正态分布的计量资料两组间比较采用成组t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验,不同时间点比较采用多配对样本秩和检验的Friedman检验。计数资料两组间比较采用χ2检验。Logistic回归分析筛选变量,建立多参数联合预测模型,采用受试者操作特征曲线(ROC曲线)评价单个指标以及联合预测模型对HBsAg清除的诊断价值。 结果 两组患者治疗前基线HBsAg水平(Z=-3.997)和治疗经历(χ2=8.221)差异均有统计学意义(P值均<0.05)。治疗中WBC(χ2=104.944)、中性粒细胞(NEUT)(χ2=132.036)、PLT(χ2=162.881)、促甲状腺激素(TSH)(χ2=83.304)逐渐下降(P值均<0.05),ALT(χ2=157.618)、AFP(χ2=159.472)逐渐升高(P值均<0.05)。治疗48周时两组患者治疗经历(OR=0.232,95%CI:0.071~0.753)、HBsAg基线值(OR=13.423,95%CI:3.276~54.997)、HBsAg治疗12周较基线下降水平(OR=0.143,95%CI:0.040~0.515)、治疗过程中ALT最大值(OR=0.986,95%CI:0.980~0.993)、治疗过程中TSH最小值(OR=3.281,95%CI:1.413~7.619)是HBsAg清除的独立影响因素(P值均<0.05)。构建HBsAg清除的联合预测模型Y=-1.603-1.462×治疗经历+2.597×HBsAg基线值-1.944×HBsAg治疗12周较基线下降水平-0.014×治疗中ALT最大值+1.188×治疗中TSH最小值。单个指标预测HBsAg清除的ROC曲线下面积(AUC)从高到低依次为治疗中ALT最大值(AUC=0.824)、HBsAg基线值(AUC=0.727)、治疗中TSH最小值(AUC=0.707)、HBsAg治疗12周较基线下降水平(AUC=0.641)、治疗经历(AUC=0.636),而联合模型的预测价值更高(AUC=0.921);联合预测模型的诊断效能与单一指标比较,显著提升(P值均<0.05)。 结论 由HBsAg基线值、HBsAg治疗12周较基线下降水平、治疗中ALT最大值、治疗中TSH最小值等构建的联合预测模型对PEG-IFN-α-2b治疗HBeAg阴性的CHB患者48周发生HBsAg清除的预测价值较高,可为筛选适合治疗的患者和预测临床治愈提供参考。 Abstract:Objective To investigate the predictive factors for the occurrence of HBsAg clearance in patients with HBeAg-negative chronic hepatitis B (CHB) receiving peginterferon alfa-2b (PEG-IFN-α-2b) treatment, analyze the effects of various indicators on the HBsAg clearance rate under different characteristics, and construct and evaluate a combined predictive model. Methods We included 125 patients with HBeAg-negative CHB at Kunming Third People’s Hospital from May 2021 to May 2023. After treatment with PEG-IFN-α-2b combined with nucleoside analogues for a course of 48 weeks, they were divided into HBsAg clearance group and HBsAg non-clearance group. Their general information and serological, biochemical, and virological indicators at different time points during treatment were recorded. Continuous data in normal distribution were compared using the t test. Continuous data in non-normal distribution were compared using the Mann-Whitney U test, and comparisons across different time points were performed using the multiple paired-sample Friedman test. Categorical data were compared using the χ2 test. A Logistic regression analysis was used to select variables to establish a combined multi-parameter predictive model. Receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic value of individual indicators and the combined predictive model for HBsAg clearance. Results Before treatment, there were significant differences in baseline HBsAg level (Z=-3.997,P<0.05) and treatment history (χ2=8.221,P<0.05) between the two groups. During treatment, gradually decreasing trends were observed in white blood cell count (χ2=104.944), neutrophil count (χ2=132.036), platelet count (χ2=162.881), and thyroid-stimulating hormone level (TSH,χ2=83.304,all P<0.05), while alanine aminotransferase (ALT,χ2=157.618) and alpha fetoprotein (χ2=159.472) showed gradually increasing trends (both P<0.05). At 48 weeks of treatment, treatment history (odds ratio [OR]=0.232, 95% confidence interval [CI]:0.071 — 0.753), baseline HBsAg level (OR=13.423,95%CI:3.276 — 54.997), the extent of decrease in HBsAg from baseline after 12 weeks of treatment (OR=0.143,95%CI:0.040 — 0.515), the maximum ALT level during treatment (OR=0.986,95%CI:0.980 — 0.993), and the minimum TSH level during treatment (OR=3.281,95%CI:1.413 — 7.619) were independent factors affecting HBsAg clearance (all P<0.05). A combined predictive model for HBsAg clearance was built:Y=-1.603-1.462×treatment history+2.597×baseline HBsAg value-1.944×the extent of HBsAg reduction from baseline after 12 weeks of treatment-0.014×the maximum ALT value during treatment+1.188×the minimum TSH value during treatment. The diagnostic value of the individual indicators for HBsAg clearance from high to low was as following: the maximum ALT value during treatment (AUC=0.824), baseline HBsAg value (AUC=0.727), the minimum TSH value during treatment (AUC=0.707), the extent of HBsAg reduction from baseline after 12 weeks of treatment (AUC=0.641), and treatment history (AUC=0.636). The combined model showed better predictive performance than the individual indicators, with the AUC being 0.921 (all P<0.05). Conclusion The combined model, constructed with baseline HBsAg value, the extent of HBsAg reduction from baseline after 12 weeks of treatment, the maximum ALT value during treatment, and the minimum TSH value during treatment, has high predictive value for the occurrence of HBsAg clearance in patients with HBeAg-negative CHB after 48 weeks of treatment with PEG-IFN-α-2b, which can provide a reference for identifying suitable patients for treatment and predicting clinical outcome. -
表 1 125例 HBeAg 阴性 CHB 患者 PEG-IFN-α-2b 联合 NUC 治疗的基线特征
Table 1. Baseline characteristics of PEG-IFN-α-2b combined with NUC treatment in 127 HBeAg negative CHB patients
指标 总计(n=125) 48周时HBsAg状态 统计值 P值 HBsAg清除组(n=37) HBsAg未清除组(n=88) 性别[例(%)] χ2 =0.136 0.712 男 88(70.40) 24(64.86) 54(61.36) 女 37(29.60) 13(35.14) 34(38.64) 年龄(岁) 34.07±12.46 35.59±13.50 33.42±12.02 t=0.885 0.378 治疗经历[例(%)] χ2=8.221 0.004 经治联合 47(37.60) 21(56.76) 26(29.55) 初治联合 78(62.40) 16(43.24) 62(70.45) 治疗方案[例(%)] χ2=2.765 0.429 PEG-IFN-α-2b联合TEV 44(35.20) 9(24.32) 35(39.77) PEG-IFN-α-2b联合TMF 38(30.40) 13(35.14) 25(28.41) PEG-IFN-α-2b联合TDF 25(20.00) 9(24.32) 16(18.18) PEG-IFN-α-2b联合TAF 18(14.40) 6(16.22) 12(13.64) HBV DNA状态 χ2=2.637 0.104 阳性 68(54.40) 16(43.24) 36(40.91) 阴性 57(45.60) 21(56.76) 52(59.09) HBsAg (log10 IU/mL) 3.04(2.03~3.89) 1.94(0.78~3.33) 3.12(2.57~3.94) Z=-3.997 <0.001 WBC (×109/L) 5.14±1.57 5.17±1.90 5.13±1.44 t=0.102 0.919 NEUT(×109/L) 2.65(1.71~3.30) 2.31(1.51~3.27) 2.65(1.75~3.29) Z=-0.430 0.667 Hb(g/L) 155.95±28.15 153.56±31.88 156.94±26.61 t=-0.605 0.546 PLT(×1012/L) 212.00(159.50~257.00) 221.00(167.00~263.00) 211.00(164.25~258.25) Z=-0.914 0.361 TBil(μmol/L) 13.00(9.45~19.20) 13.60(9.80~19.40) 12.75(9.55~17.83) Z=-0.357 0.721 ALT(U/L) 46.00(23.00~105.00) 49.00(23.00~125.00) 43.00(22.25~99.50) Z=-0.281 0.778 TSH(μIU/mL) 2.67(2.16~3.87) 2.72(2.16~3.69) 2.62(2.15~4.15) Z=-0.327 0.744 AFP(ng/mL) 2.80(2.14~1.05) 2.92(1.97~4.74) 2.78(2.11~3.89) Z=-0.149 0.882 LSM(kPa) 6.37(4.80~8.95) 6.60(4.50~8.90) 6.20(4.70~9.30) Z=-0.008 0.994 表 2 HBeAg阴性CHB患者经PEG-IFN-α-2b联合NUC治疗前后生化指标的变化
Table 2. Changes of biochemical indexes of HBeAg negative CHB patients before and after PEG-IFN-α-2b combined with NUC treatment
指标 基线 治疗12周 治疗24周 χ2值 P值 WBC(×109/L) 5.16(4.21~5.94) 3.52(2.85~4.51)1) 3.25(2.61~4.33)1) 104.944 <0.001 NEUT(×109/L) 2.65(1.71~3.30) 1.47(1.16~1.85)1) 1.05(0.82~1.51)1)2) 132.036 <0.001 PLT(×1012/L) 212.0(159.50~257.00) 132.00(99.00~159.50)1) 98.00(80.00~131.00)1)2) 162.881 <0.001 ALT(U/L) 46.00(23.00~105.00) 52.00(39.50~94.50) 100.00(61.00~158.50)1)2) 157.618 <0.001 TSH(μIU/mL) 2.67(2.16~3.87) 2.14(1.25~3.25) 1.59(0.89~2.26)1)2) 83.304 <0.001 AFP(ng/mL) 2.80(2.14~4.05) 4.52(2.70~6.50)1) 8.16(5.32~13.81)1)2) 159.472 <0.001 注:与基线比较,1)P<0.05;与治疗12周比较,2)P<0.05。
表 3 单因素和多因素Logistic回归分析HBeAg阴性CHB患者治疗48周HBsAg清除的影响因素
Table 3. Univariate and multivariate Logistic regression analysis of influencing factors on HBsAg clearance in HBeAg negative CHB patients after 48 weeks of treatment
指标 单因素分析 多因素分析 β值 P值 OR 95%CI β值 P值 OR 95%CI 性别(男/女) -0.368 0.381 0.692 0.304~1.575 年龄(岁) -0.014 0.375 0.986 0.956~1.017 治疗经历(经治联合/初治联合) -1.141 0.005 0.320 0.144~0.708 -1.462 0.015 0.232 0.071~0.753 HBV DNA状态(阴性/阳性) -0.640 0.107 0.527 0.243~1.147 HBsAg基线值(log10 IU/mL) 0.743 <0.001 2.102 1.481~2.983 2.597 <0.001 13.423 3.276~54.997 HBsAg 12周较基线下降水平(log10 IU/mL) 0.388 0.011 1.473 1.092~1.988 -1.944 0.003 0.143 0.040~0.515 HBsAg 24周较基线下降水平(log10 IU/mL) 0.605 <0.001 1.832 1.317~2.548 0.370 0.135 1.447 0.892~2.349 治疗中WBC最小值(×109/L) 0.186 0.346 1.204 0.818~1.772 治疗中NEUT最小值(×109/L) 1.243 0.081 3.468 0.857~14.030 治疗中PLT最小值(×1012/L) 0.010 0.108 1.010 0.998~1.022 治疗中ALT最大值(U/L) -0.008 0.001 0.992 0.988~0.997 -0.014 <0.001 0.986 0.980~0.993 治疗中TSH最小值(μIU/mL) 1.000 0.001 2.718 1.476~5.003 1.188 0.006 3.281 1.413~7.619 治疗中AFP最大值(ng/mL) -0.032 0.071 0.968 0.935~1.003 表 4 单个指标以及联合预测模型诊断价值比较
Table 4. Comparison of diagnostic value between individual indicators and combined predictive model
指标 AUC 截断值 约登指数 敏感度(%) 特异度(%) 95%CI Z值 P值 治疗经历(经治联合/初治联合) 0.636 0.273 56.80 70.50 0.527~0.745 -5.794 <0.001 HBsAg基线值(log10 IU/mL) 0.727 2.13 0.443 56.80 87.50 0.624~0.830 -3.766 <0.001 HBsAg 12周较基线下降水平(log10 IU/mL) 0.641 1.81 0.348 56.80 75.00 0.535~0.748 -4.911 <0.001 治疗中ALT最大值(U/L) 0.824 152.00 0.594 73.00 86.40 0.612~0.802 -2.077 0.006 治疗中TSH最小值(μIU/mL) 0.707 1.26 0.427 75.50 67.00 0.612~0.802 -4.133 <0.001 联合预测 0.921 0.758 83.80 92.00 0.866~0.975 表 5 不同特征下 HBsAg 的清除率比较
Table 5. Comparison of the HBsAg clearance rates in CHB patients with different characteristics
指标 例数 HBsAg清除 HBsAg未清除 χ2值 P值 治疗经历[例(%)] 8.221 0.004 经治联合 47 21(44.68) 26(55.32) 初治联合 78 16(20.51) 62(79.49) HBsAg基线值[例(%)] 26.787 <0.001 ≤2.13 log10 IU/mL 32 21(65.63) 11(34.37) >2.13 log10 IU/mL 93 16(17.20) 77(82.80) HBsAg 治疗12周较基线下降水平[例(%)] 1.266 0.261 ≥1.81 log10 IU/mL 82 27(32.93) 55(67.07) <1.81 log10 IU/mL 43 10(23.26) 33(76.74) 治疗中ALT最大值[例(%)] 46.073 <0.001 ≥152 U/L 40 28(70.00) 12(30.00) <152 U/L 85 9(10.59) 76(89.41) 治疗中TSH最小值[例(%)] 18.112 <0.001 ≤1.26 μIU/mL 58 28(48.28) 30(51.72) >1.26 μIU/mL 67 9(13.43) 58(86.57) -
[1] YANG XA, ZHANG K, XU QH, et al. Interferon add-on therapy increased clinical cure significantly for interferon-experienced chronic hepatitis B patients with low HBsAg[J]. Front Immunol, 2022, 13: 997608. DOI: 10.3389/fimmu.2022.997608. [2] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update[J]. J Clin Hepatol, 2015, 31( 12): 1941- 1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31( 12): 1941- 1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [3] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. J Prac Hepatol, 2023, 26( 3): Suppl. 1- 22. DOI: 10.3969/j.issn.1672-5069.2023.040.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 实用肝脏病杂志, 2023, 26( 3): 后插1-后插 22. DOI: 10.3969/j.issn.1672-5069.2023.03.040. [4] OGUNNAIKE M, DAS S, RAUT SS, et al. Chronic hepatitis B infection: New approaches towards cure[J]. Biomolecules, 2023, 13( 8): 1208. DOI: 10.3390/biom13081208. [5] YAN Y, CHANTSALMAA D, LYU CY, et al. Predictive value of baseline serum marker levels for the effect of interferon therapy in patients with chronic hepatitis B[J]. J Sichuan Univ Med Sci, 2024, 55( 2): 383- 390. DOI: 10.12182/20240360105.阎岩, Chantsalmaa Davgadorj, 吕春燕, 等. 慢性乙型肝炎患者的血清标志物基线水平对干扰素治疗效果的预测价值[J]. 四川大学学报(医学版), 2024, 55( 2): 383- 390. DOI: 10.12182/20240360105. [6] ZHANG WH, ZHANG DZ, DOU XG, et al. Consensus on pegylated interferon alpha in treatment of chronic hepatitis B[J]. Chin J Hepatol, 2017, 25( 9): 678- 686. DOI: 10.3760/cma.j.issn.1007-3418.2017.09.007.张文宏, 张大志, 窦晓光, 等. 聚乙二醇干扰素α治疗慢性乙型肝炎专家共识[J]. 中华肝脏病杂志, 2017, 25( 9): 678- 686. DOI: 10.3760/cma.j.issn.1007-3418.2017.09.007. [7] LI YP, LIU CR, HE L, et al. Hepatitis B cure: Current situation and prospects[J]. World J Hepatol, 2024, 16( 6): 900- 911. DOI: 10.4254/wjh.v16.i6.900. [8] FARAG MS, van CAMPENHOUT MJH, SONNEVELD MJ, et al. Addition of PEG-interferon to long-term nucleos(t)ide analogue therapy enhances HBsAg decline and clearance in HBeAg-negative chronic hepatitis B: Multicentre Randomized Trial(PAS Study)[J]. J Viral Hepat, 2024, 31( 4): 197- 207. DOI: 10.1111/jvh.13918. [9] WU FP, YANG Y, LI M, et al. Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen≤1 500 IU/mL: An observational study[J]. World J Gastroenterol, 2020, 26( 13): 1525- 1539. DOI: 10.3748/wjg.v26.i13.1525. [10] LI K, NING HB, JIN HM, et al. Effect of pegylated interferon α-2b on serum HBsAg clearance rate in treatment of patients with chronic hepatitis B[J]. J Clin Hepatol, 2023, 39( 8): 1819- 1824. DOI: 10.3969/j.issn.1001-5256.2023.08.009.李宽, 宁会彬, 靳慧鸣, 等. 聚乙二醇干扰素α-2b治疗慢性乙型肝炎患者血清HBsAg清除率的效果分析[J]. 临床肝胆病杂志, 2023, 39( 8): 1819- 1824. DOI: 10.3969/j.issn.1001-5256.2023.08.009. [11] WANG JL, XI DY, YAN XB, et al. Predictors of HBsAg clearance in HBeAg-negative chronic hepatitis B patients treated with pegylated interferon α-2b and the construction of a nomogram model[J]. J Clin Hepatol, 2023, 39( 12): 2809- 2816. DOI: 10.3969/j.issn.1001-5256.2023.12.010.王佳露, 席德扬, 颜学兵, 等. 聚乙二醇干扰素α-2b治疗HBeAg阴性慢性乙型肝炎患者实现HBsAg清除的预测因素及列线图构建[J]. 临床肝胆病杂志, 2023, 39( 12): 2809- 2816. DOI: 10.3969/j.issn.1001-5256.2023.12.010. [12] ZANG HY, LI WN, LIU SS, et al. Predictive factors for functional cure after sequential therapy with nucleos(t)ide analogues and pegylated interferon Alfa-2b in treatment of chronic hepatitis B[J]. J Clin Hepatol, 2023, 39( 2): 299- 306. DOI: 10.3969/j.issn.1001-5256.2023.02.008.臧海洋, 李伟娜, 刘守胜, 等. 核苷(酸)类似物序贯派格宾治疗慢性乙型肝炎实现功能性治愈的预测因素[J]. 临床肝胆病杂志, 2023, 39( 2): 299- 306. DOI: 10.3969/j.issn.1001-5256.2023.02.008. [13] HE XJ, LONG YZ, ZHOU J, et al. Serum hepatitis B virus RNA monitoring pegylated interferon therapy nucleos(t) ide analogues in the treatment of low viral load in patients with chronic hepatitis B curative effect[J]. Clin J Med Offic, 2023, 51( 10): 1091- 1095. DOI: 10.16680/j.1671-3826.2023.10.27.贺潇瑾, 龙云铸, 周娟, 等. 血清乙型肝炎病毒RNA监测聚乙二醇干扰素治疗核苷(酸)类似物经治低病毒载量慢性乙型肝炎患者疗效[J]. 临床军医杂志, 2023, 51( 10): 1091- 1095. DOI: 10.16680/j.1671-3826.2023.10.27. [14] LI YY, YANG SQ, LI C, et al. Efficacy of short-term Peg-IFN α-2b treatment in chronic hepatitis B patients with ultra-low HBsAg levels: A retrospective cohort study[J]. Virol J, 2024, 21( 1): 231. DOI: 10.1186/s12985-024-02512-w. DOI: 10.3969/j.issn.1001-5256.2023.02.008. [15] ZHONG WT, YAN LZ, ZHU YG, et al. A high functional cure rate was induced by pegylated interferon alpha-2b treatment in postpartum hepatitis B e antigen-negative women with chronic hepatitis B virus infection: An exploratory study[J]. Front Cell Infect Microbiol, 2024, 14: 1426960. DOI: 10.3389/fcimb.2024.1426960. [16] IANNAZZO S, COCO B, BRUNETTO MR, et al. Individualized treatment of HBeAg-negative chronic hepatitis B using pegylated interferon-α2a as first-line and week-12 HBV DNA/HBsAg stopping rule: A cost-effectiveness analysis[J]. Antivir Ther, 2013, 18( 4): 623- 633. DOI: 10.3851/IMP2555. [17] GU LL, HU R, DOU YM, et al. Predictors associated with functional cure in HBeAg-negative chronic hepatitis B patients treated with pegylated interferon α-2b[J]. Chin Hepatol, 2023, 28( 3): 313- 319. DOI: 10.14000/j.cnki.issn.1008-1704.2023.03.014.顾琳琳, 胡瑞, 窦宇明, 等. 聚乙二醇干扰素α-2b治疗HBeAg阴性慢性乙型肝炎实现临床治愈的预测因素分析[J]. 肝脏, 2023, 28( 3): 313- 319. DOI: 10.14000/j.cnki.issn.1008-1704.2023.03.014. [18] WONG D, LITTLEJOHN M, EDWARDS R, et al. ALT flares during nucleotide analogue therapy are associated with HBsAg loss in genotype A HBeAg-positive chronic hepatitis B[J]. Liver Int, 2018, 38( 10): 1760- 1769. DOI: 10.1111/liv.13716. [19] CHIEN RN, LIAW YF. Re-treatment for severe hepatitis flare in HBeAg-negative chronic hepatitis B: An appraisal with combined HBsAg/ALT kinetics[J]. J Viral Hepat, 2020, 27( 5): 544- 547. DOI: 10.1111/jvh.13253. [20] WU LL, GAO ZL. Predictive factors for HBsAg-negative seroconversion in chronic hepatitis B after antiviral therapy[J]. Chin J Hepatol, 2024, 32( 2): 186- 192. DOI: 10.3760/cm3.j.cn501113-20231213-00278.吴丽丽, 高志良. 抗病毒治疗后慢性乙型肝炎HBsAg阴转的预测因子[J]. 中华肝脏病杂志, 2024, 32( 2): 186- 192. DOI: 10.3760/cma.j.cn501113-20231213-00278. [21] TANG QQ, YE J, ZHANG YF, et al. Establishment of a multi-parameter prediction model for the functional cure of HBeAg-negative chronic hepatitis B patients treated with pegylated interferonα and decision process based on response-guided therapy strategy[J]. BMC Infect Dis, 2023, 23( 1): 456. DOI: 10.1186/s12879-023-08443-1. [22] MA ZX, QIN YL, JIA YD, et al. Thyroid dysfunction incidence and risk factors in Chinese chronic hepatitis B patients treated with pegylated interferon alpha: A long-term follow-up study[J]. J Viral Hepat, 2022, 29( 6): 412- 419. DOI: 10.1111/jvh.13667. [23] HUANG FT, LIN YM, RAO KM, et al. Effect of a—interferon in treating chronic hepatitis B on liver function,hepatitis B surface antigen and thyroid function[J]. Lab Med Clin, 2024, 21( 19): 2920- 2924. DOI: 10.3969/j.issn.1672-9455.2024.19.0274.皇甫彤, 蔺咏梅, 饶珂萌, 等. α-干扰素治疗慢性乙型肝炎对患者肝功能、乙型肝炎表面抗原及甲状腺功能的影响[J]. 检验医学与临床, 2024, 21( 19): 2920- 2924. DOI: 10.3969/j.issn.1672-9455.2024.19.0274. [24] MU H, XU DQ, LIU CY, et al. Relationship between HBsAg and TSH during interferon treatment in hepatitis B patients with low HBsAg level[J]. Chongqing Med J, 2024, 53( 18): 2826- 2829. DOI: 10.3969/j.issn.1671-8348.2024.18.020.木唤, 许丹青, 刘春云, 等. 低HBsAg水平乙型肝炎患者使用干扰素治疗期间HBsAg与TSH的关系[J]. 重庆医学, 2024, 53( 18): 2826- 2829. DOI: 10.3969/j.issn.1671-8348.2024.18.020. -



 PDF下载 ( 1070 KB)
PDF下载 ( 1070 KB)

 下载:
下载:


