鸢尾黄素(TEC)对肝癌细胞活力、迁移和凋亡的影响及其机制
DOI: 10.12449/JCH251019
Effect of tectorigenin on the viability, migration, and apoptosis of hepatoma cells and its mechanism
-
摘要:
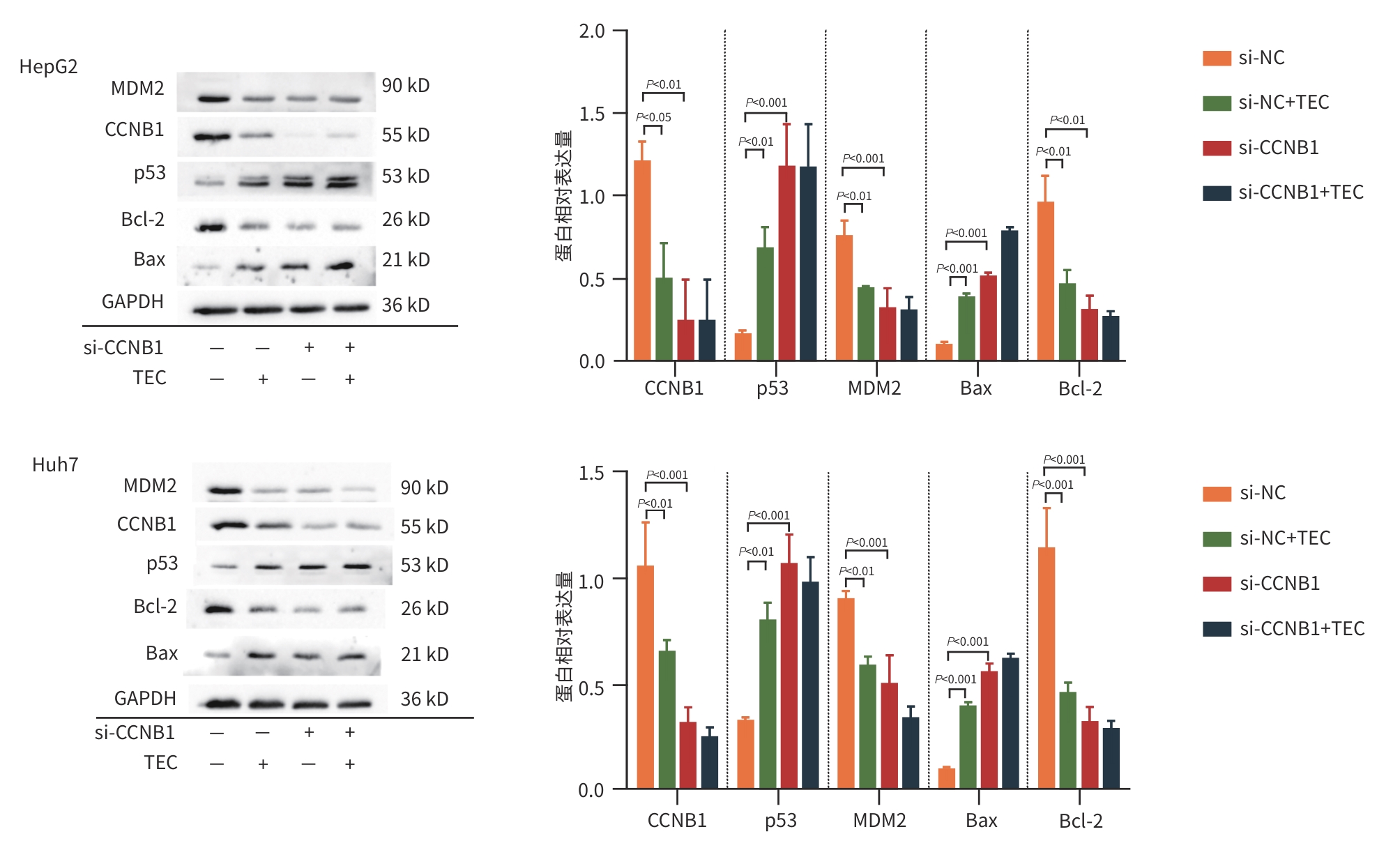
目的 观察蓝莓单体鸢尾黄素(TEC)对肝癌细胞系HepG2、Huh7细胞的影响,并探讨其作用机制。 方法 从蓝莓中提取纯化TEC;利用生物信息学进行靶基因及信号通路筛选;分别采用HepG2、Huh7细胞系,并设置4个TEC浓度处理组(0、30、60和90 μg/mL)进行实验。通过CCK-8法检测细胞活力;划痕实验、Transwell实验评估迁移能力;流式细胞术检测凋亡率;Western Blot检测CCNB1、p53、MDM2、Bax、Bcl-2和active-Caspase 3蛋白表达。构建CCNB1低表达(共5组:NC组、si-NC组、si-NC+TEC组、si-CCNB1组和si-CCNB1+TEC组)和过表达(共4组:OE-NC组、OE-NC+TEC组、OE-CCNB1组和OE-CCNB1+TEC组)细胞模型验证靶点。计量资料多组间比较采用单因素方差分析或双因素方差分析,进一步两两比较采用LSD-t检验。癌组织及癌旁组织间基因表达量的比较采用Wilcoxon符号秩和检验。 结果 在HepG2、Huh7细胞中,同一TEC浓度下,TEC干预24 h时细胞存活率显著低于12 h和48 h(P值均<0.05);在干预时间为24 h时,TEC 90 μg/mL浓度组的细胞存活率均显著低于其他浓度组(P值均<0.05)。故采用TEC浓度为90 μg/mL、干预时长为24 h进行后续实验。与TEC 0 μg/mL组相比,30、60、90 μg/mL浓度组细胞的迁移数量及划痕愈合率均显著降低(P值均<0.05);与NC组及si-NC组相比,HepG2和Huh7细胞si-NC+TEC组及si-CCNB1组的迁移细胞数目、划痕愈合率均出现了明显的下降(P值均<0.05)。与NC组及si-NC组相比,HepG2和Huh7细胞si-NC+TEC组、si-CCNB1组的细胞凋亡率均显著上升(P值均<0.05)。在HepG2细胞中,与0 μg/mL组相比,30、60、90 μg/mL浓度组均表现出CCNB1和Bcl-2蛋白表达水平的下调(P值均<0.05),60和90 μg/mL浓度组p53、Bax、active-Caspase 3蛋白表达水平上调(P值均<0.001),同时伴随MDM2蛋白表达的降低(P值均<0.05)。在Huh7细胞中,与0 μg/mL组相比,30、60和90 μg/mL组CCNB1蛋白表达降低(P值均<0.01);60和90 μg/mL浓度组p53和Bax蛋白表达显著上调,MDM2蛋白下调(P值均<0.05);90 μg/mL浓度组Bcl-2蛋白表达下调、active-Caspase 3蛋白表达上调(P值均<0.01)。与si-NC组相比,HepG2和Huh7细胞中si-NC+TEC组及si-CCNB1组CCNB1、MDM2、Bcl-2蛋白的表达水平均显著下调,p53和Bax蛋白的表达水平均显著上调(P值均<0.05)。与OE-NC组相比,HepG2和Huh7细胞OE-NC+TEC组CCNB1、MDM2显著下调,p53蛋白水平上调(P值均<0.05),而OE-CCNB1组的CCNB1和MDM2蛋白表达水平均显著上调,p53蛋白表达水平则显著下调(P值均<0.05);OE-CCNB1组与OE-CCNB1+TEC组间CCNB1、MDM2及p53的蛋白表达水平比较差异均无统计学意义(P值均>0.05)。 结论 TEC可在体外抑制HepG2和Huh7细胞增殖、迁移,促进细胞凋亡,其机制可能是通过下调CCNB1表达,激活p53信号通路。 Abstract:Objective To investigate the effect of blueberry-derived tectorigenin (TEC) on hepatocellular carcinoma cell lines HepG2 and Huh7 and its mechanism. Methods TEC was extracted from blueberries and purified, and a bioinformatics analysis was performed to identify potential target genes and signaling pathways. HepG2 and Huh7 cell lines were used and divided into 0, 30, 60, and 90 μg/mL groups according to the concentration of TEC. CCK-8 assay was used to measure cell viability; wound healing assay and Transwell assay were used to assess the migration ability of cells; flow cytometry was used to measure cell apoptosis rate; Western Blot was used to measure the protein expression levels of CCNB1, p53, MDM2, Bax, Bcl-2, and active-Caspase 3. Cell models with low CCNB1 expression (NC group, si-NC group, si-NC+TEC group, si-CCNB1 group, and si-CCNB1+TEC group) and CCNB1 overexpression (OE-NC group, OE-NC+TEC group, OE-CCNB1 group, and OE-CCNB1+TEC group) were established to validate the targets. A one-way analysis of variance or two factors analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. The Wilcoxon signed rank sum test was used to compare the expression levels of genes between cancer tissue and paracancerous tissue. Results In HepG2 and Huh7 cells under the same concentration of TEC, cell viability at 24 hours of TEC intervention was significantly lower than that at 12 and 48 hours (all P<0.05), and at 24 hours of intervention, the TEC 90 μg/mL group had a significantly lower cell viability than the other groups (all P<0.05). Therefore, TEC intervention for 24 hours at a concentration of 90 μg/mL was used for subsequent studies. Compared with the TEC 0 μg/mL group, the 30, 60, and 90 μg/mL groups had significant reductions in the number of migrated cells and wound healing rate (all P<0.05), and compared with the NC group and si-NC group, the si-NC+TEC group and the si-CCNB1 group for HepG2 and Huh7 cells had significant reductions in the number of migrated cells and wound healing rate (all P<0.05). Compared with the NC group and si-NC group, the si-NC+TEC group and the si-CCNB1 group for HepG2 and Huh7 cells had a significant increase in cell apoptosis rate (all P<0.05). For HepG2 cells, compared with the 0 μg/mL group, the 30, 60, and 90 μg/mL groups had significant reductions in the protein expression levels of CCNB1 and Bcl-2 (all P<0.05), and the 60 and 90 μg/mL groups had significant increases in the protein expression levels of p53, Bax, and active-Caspase 3 (all P<0.001) and a significant reduction in the protein expression level of MDM2 (both P<0.05). For Huh7 cells, compared with the 0 μg/mL group, the 30, 60, and 90 μg/mL groups had a significant reduction in the protein expression level of CCNB1 (all P<0.01); the 60 and 90 μg/mL groups had significant increases in the protein expression levels of p53 and Bax and a significant reduction in the protein expression level of MDM2 (all P<0.05); the 90 μg/mL group had a significant reduction in the protein expression level of Bcl-2 and a significant increase in the protein expression level of active-Caspase 3 (both P<0.01). Compared with the si-NC group, the si-NC+TEC group and the si-CCNB1 group for HepG2 and Huh7 cells had significant reductions in the protein expression levels of CCNB1, MDM2, and Bcl-2 and significant increases in the protein expression levels of p53 and Bax (all P<0.05). Compared with the OE-NC group, the OE-NC+TEC group for HepG2 and Huh7 cells had significant reductions in the protein expression levels of CCNB1 and MDM2 and a significant increase in the protein expression level of p53 (all P<0.05), while the OE-CCNB1 group had significant increases in the protein expression levels of CCNB1 and MDM2 and a significant reduction in the protein expression level of p53 (all P<0.05), and there were no significant differences in the protein expression level of CCNB1, MDM2, and p53 between the OE-CCNB1 group and the OE-CCNB1+TEC group (all P>0.05). Conclusion TEC can inhibit the proliferation and migration of HepG2 and Huh7 cells and promote their apoptosis in vitro, possibly by downregulating the expression of CCNB1 and activating the p53 signaling pathway. -
Key words:
- Anthocyanins /
- Liver Neoplasms /
- Hep G2 Cells /
- Cyclin B1
-
注: a,GSE101685、GSE6764、GSE121248数据集中肝癌差异表达基因KEGG富集分析;b,肝癌组织和癌旁组织关键基因表达差异热图;c,数据集合中上调的差异基因与细胞周期相关基因交集;d,HepG2、Huh7细胞CCNB1、CDK1、CCNE2、CDKN2C、MCM6、CDKN2A mRNA相对表达量(与CCNB1相比,***P<0.001,n=3);e,CCNB1在肝癌组织与癌旁组织中的表达差异;f,GTEx数据库中CCNB1表达与肝癌患者的总生存期及无病生存期的关系。
图 2 CCNB1在肝癌组织中高表达
Figure 2. CCNB1 is highly expressed in hepatocellular carcinoma tissues
表 1 HepG2和Huh7细胞经不同浓度TEC干预后各组存活率比较
Table 1. The effect of different concentrations of TEC on the survival rate of HepG2 and Huh7 cells
TEC浓度
(μg/mL)细胞存活率 12 h 24 h 48 h HepG2 0 1.0001) 1.0001) 1.0001) 30 0.829±0.1021)2) 0.647±0.0421) 0.911±0.0901)2) 60 0.749±0.0871)2) 0.588±0.0641) 0.839±0.0342) 90 0.585±0.0622) 0.516±0.055 0.742±0.0962) Huh7 0 1.0001) 1.0001) 1.000 30 0.937±0.0521)2) 0.803±0.0291) 0.983±0.0342) 60 0.843±0.0382) 0.752±0.0571) 0.987±0.0512) 90 0.812±0.1312) 0.734±0.063 0.913±0.0922) 注:与同一时间点内TEC浓度为90 μg/mL组比较,1) P<0.05;与同一TEC浓度内干预时长为24 h比较,2) P<0.05。
-
[1] TOH MR, WONG EYT, WONG SH, et al. Global epidemiology and genetics of hepatocellular carcinoma[J]. Gastroenterology, 2023, 164( 5): 766- 782. DOI: 10.1053/j.gastro.2023.01.033. [2] SILVA S, COSTA EM, VEIGA M, et al. Health promoting properties of blueberries: A review[J]. Crit Rev Food Sci Nutr, 2020, 60( 2): 181- 200. DOI: 10.1080/10408398.2018.1518895. [3] ZHAN W, LIAO X, YU L, et al. Effects of blueberries on migration, invasion, proliferation, the cell cycle and apoptosis in hepatocellular carcinoma cells[J]. Biomed Rep, 2016, 5( 5): 579- 584. DOI: 10.3892/br.2016.774. [4] RONG J, FU F, HAN CX, et al. Tectorigenin: A review of its sources, pharmacology, toxicity, and pharmacokinetics[J]. Molecules, 2023, 28( 15): 5904. DOI: 10.3390/molecules28155904. [5] JIANG CP, DING H, SHI DH, et al. Pro-apoptotic effects of tectorigenin on human hepatocellular carcinoma HepG2 cells[J]. World J Gastroenterol, 2012, 18( 15): 1753- 1764. DOI: 10.3748/wjg.v18.i15.1753. [6] YEH LT, HSU LS, CHUNG YH, et al. Tectorigenin inhibits glioblastoma proliferation by G0/G1 cell cycle arrest[J]. Medicina(Kaunas), 2020, 56( 12): 681. DOI: 10.3390/medicina56120681. [7] ZENG LW, YUAN SF, SHEN JL, et al. Suppression of human breast cancer cells by tectorigenin through downregulation of matrix metalloproteinases and MAPK signaling in vitro[J]. Mol Med Rep, 2018, 17( 3): 3935- 3943. DOI: 10.3892/mmr.2017.8313. [8] FANG R, HOUGHTON PJ, HYLANDS PJ. Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines[J]. J Ethnopharmacol, 2008, 118( 2): 257- 263. DOI: 10.1016/j.jep.2008.04.006. [9] YANG YJ, KE TY, LIU SX, et al. Synergistic sensitization of apatinib mesylate and radiotherapy on hepatocarcinoma cells in vitro[J]. J Jilin Univ(Med Edit), 2024, 50( 4): 1009- 1015. DOI: 10.13481/j.1671-587X.2024-04015.杨永净, 柯天洋, 刘士新, 等. 甲磺酸阿帕替尼联合放疗对肝癌HepG2细胞的体外协同增敏作用[J]. 吉林大学学报(医学版), 2024, 50( 4): 1009- 1015. DOI: 10.13481/j.1671-587X.202404015. [10] NAEEM A, HU PY, YANG M, et al. Natural products as anticancer agents: Current status and future perspectives[J]. Molecules, 2022, 27( 23): 8367. DOI: 10.3390/molecules27238367. [11] ZHAN G, PAN LQ, TU K, et al. Antitumor, antioxidant, and nitrite scavenging effects of Chinese water chestnut(Eleocharis dulcis) peel flavonoids[J]. J Food Sci, 2016, 81( 10): H2578- H2586. DOI: 10.1111/1750-3841.13434. [12] KIM EM, JUNG CH, KIM J, et al. The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting bcl-2 family proteins[J]. Cancer Res, 2017, 77( 11): 3092- 3100. DOI: 10.1158/0008-5472.CAN-16-2098. [13] GAVET O, PINES J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis[J]. Dev Cell, 2010, 18( 4): 533- 543. DOI: 10.1016/j.devcel.2010.02.013. [14] FANG YF, YU H, LIANG X, et al. Chk1-induced CCNB1 overexpression promotes cell proliferation and tumor growth in human colorectal cancer[J]. Cancer Biol Ther, 2014, 15( 9): 1268- 1279. DOI: 10.4161/cbt.29691. [15] LUNDGREN C, AHLIN C, HOLMBERG L, et al. Cyclin E1 is a strong prognostic marker for death from lymph node negative breast cancer. A population-based case-control study[J]. Acta Oncol, 2015, 54( 4): 538- 544. DOI: 10.3109/0284186X.2014.965274. [16] ZHOU L, LI J, ZHAO YP, et al. The prognostic value of Cyclin B1 in pancreatic cancer[J]. Med Oncol, 2014, 31( 9): 107. DOI: 10.1007/s12032-014-0107-4. [17] ZOU YP, RUAN SY, JIN L, et al. CDK1, CCNB1, and CCNB2 are prognostic biomarkers and correlated with immune infiltration in hepatocellular carcinoma[J]. Med Sci Monit, 2020, 26: e925289. DOI: 10.12659/MSM.925289. [18] WANG HL, GUO M, WEI HD, et al. Targeting p53 pathways: Mechanisms, structures, and advances in therapy[J]. Signal Transduct Target Ther, 2023, 8( 1): 92. DOI: 10.1038/s41392-023-01347-1. [19] KOO N, SHARMA AK, NARAYAN S. Therapeutics targeting p53-MDM2 interaction to induce cancer cell death[J]. Int J Mol Sci, 2022, 23( 9): 5005. DOI: 10.3390/ijms23095005. [20] LOU J, ZHAO L, ZHU YJ, et al. Effect of Fuzheng Ruanjian Anticancer Formula on malignant biological behaviors of hepatocellulars carcinoma HepG2 cells by regulating Akt/MDM2/P53 signaling pathway[J]. J Jilin Univ(Med Edit), 2024, 50( 6): 1654- 1663. DOI: 10.13481/j.1671-587X.20-240619.娄静, 赵雷, 朱岩洁, 等. 扶正软坚抗癌方调控Akt/MDM2/P53信号通路对肝癌HepG2细胞恶性生物学行为的影响[J]. 吉林大学学报(医学版), 2024, 50( 6): 1654- 1663. DOI: 10.13481/j.1671-587X.20240619. [21] YUAN JP, YAN RL, KRÄMER A, et al. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells[J]. Oncogene, 2004, 23( 34): 5843- 5852. DOI: 10.1038/sj.onc.1207757. [22] ZHANG H, ZHANG X, LI X, et al. Effect of CCNB1 silencing on cell cycle, senescence, and apoptosis through the p53 signaling pathway in pancreatic cancer[J]. J Cell Physiol, 2018, 234( 1): 619- 631. DOI: 10.1002/jcp.26816. [23] XIA P, ZHANG H, XU KQ, et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma[J]. Cell Death Dis, 2021, 12( 7): 691. DOI: 10.1038/s41419-021-03973-5. -



 PDF下载 ( 85378 KB)
PDF下载 ( 85378 KB)


 下载:
下载: